A little over a year ago, medical device startup Contraline made headlines for becoming the first company to implant a male contraceptive gel into humans in a clinical trial. Fast forward to now, and the nonhormonal hydrogel has been implanted into the vas deferens of more than 20 men — and the trial data looks good.
On Thursday, Virginia-based Contraline released promising data from the clinical trial it’s conducting to test the efficacy of Adam, its male birth control product. The hydrogel seems to be doing a good job of blocking the flow of sperm to the vas deferens, and no serious adverse events have been reported.
When the trial began in November 2022, Contraline CEO Kevin Eisenfrats told MedCity News that Adam is sometimes referred to as the “IUD for men” because it seeks to be a long-lasting yet reversible birth control method.
That’s pretty much where the analogy stops, though — Adam is nonhormonal and comes in gel form. But similar to an IUD, Adam can be implanted at a doctor’s office visit.
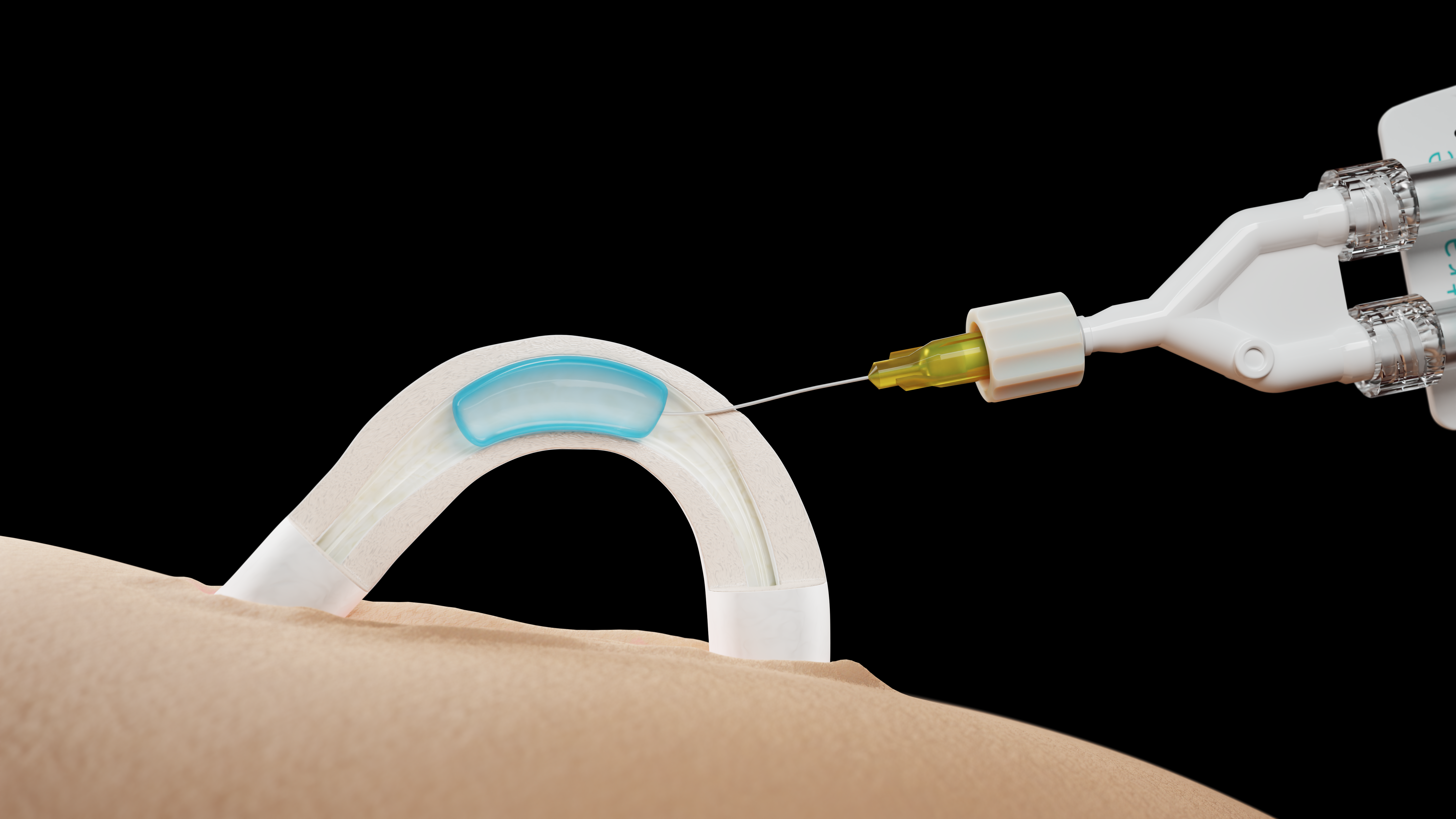
The office procedure is conducted under local anesthesia and takes less than 20 minutes, Eisenfrats said. Using a noninvasive, no-scalpel method, the doctor injects the hydrogel into the patient’s vas deferens. Once sperm is blocked from traveling through the vas deferens, a man does not produce sperm in his ejaculate, and is therefore unable to fertilize an egg.
Doctors have now implanted the hydrogel into 23 men. Next month, after two more men undergo the procedure, Contraline’s first in-human efficacy trial will reach its full enrollment at 25 patients. The trail is being conducted across three medical centers in Australia.

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
All 23 procedures conducted so far have been successful — meaning the doctor was able to exteriorize and cannulate the vas deferens, as well as implant the hydrogel using Contraline’s proprietary injector device.
Contraline has been keeping a close eye on its trial participants and plans to do so for the next couple years, Eisenfrats noted. There have been no reported cases of any serious adverse events. Some patients have reported things like light bruising or swelling in the couple days following their procedure, but these cases have been mild and went away quickly, he said.
Not only does Adam seem pretty easy to implant, but it is also appearing quite effective at its goal: to prevent the release of sperm. Contraline’s trial data shows that within 30 days of implantation, participants showed a 99.6-100% reduction in motile sperm.
“We’re really seeing phenomenal results in terms of the reduction and sperm count and motility, which signifies that this is a very effective male contraceptive,” Eisenfrats declared.
He added that Contraline has seen a vast amount of interest in its product, from men of all ages and life circumstances.
When the startup began advertising for its trial, it intentionally made the application survey lengthy and full of detailed questions so it could weed out anyone who wasn’t serious about wanting to try Adam, Eisenfrats explained. Even so, more than 1,500 men completed the application. He noted that these men came from various walks of life — some were in their early 20s, some were middle-aged, some were in long-term relationships, some were single, some were childless, and some had already become fathers.
There’s a clear demand for a product like Adam, and Eisenfrats believes that the new clinical data demonstrates that Contraline is on track to put the first reversible, nonhormonal male contraceptive on the market.
There’s not a lot of competition in the male birth control space. Eisenfrats identified Vasalgel — a male contraceptive gel that the nonprofit Parsemus Foundation has been developing for years — as Contraline’s only direct competitor. The foundation recently partnered with biotech NEXT Life Sciences to develop Vasalgel under the name Plan A, but no data has been released proving that the product is effective or reversible.
Now that Contraline has released in-human clinical trial data showing promising results about Adam’s effectiveness, the startup is getting ready to launch another trial this year — one that will test the hydrogel’s reversibility.
“Reversibility is a really key value proposition. Men want to know that they can get it removed at any point in time, that their fertility will come back to normal levels, and that the reversal procedure is easy. So, we’re starting to do a small proof of concept trial specifically on reversibility that will start in mid-2024,” Eisenfrats explained.
After that, Contraline plans to initiate some pivotal trials in the U.S., he said.
The company’s goal is to file for an investigational device exemption from the FDA by the end of the year, Eisenfrats stated. This would allow the startup to begin the clinical testing its needs for Adam to eventually be approved by the FDA.
“That pivotal trial will likely be starting next year. With the pivotal trials starting in 2025, we’d love to get approval for Adam in the U.S. around 2026 or 2027,” he declared.
Photo: Contraline