
Non-profit Sues FDA, Requesting the Agency Withdraw Approval for Abortion Drugs
The group complains that the FDA never studied the safety of the drugs under the labeled conditions of use.

The group complains that the FDA never studied the safety of the drugs under the labeled conditions of use.
Here's a compilation of myths and realities about Corrective Action and Preventive Action (CAPA), the system for addressing systemic quality issues for medical device companies.

Corrective Action and Preventive Action (CAPA), a system for addressing systemic quality issues for medical device companies, is frequently overused, which can overburden businesses with unnecessary work, or when underutilized, trigger FDA warning letters.

Trump also called for reforming the "slow and burdensome" FDA drug approval process.

Dr. Robert Califf, who stepped down last month, offers five tips about keeping Americans safe — and making sure drugs actually work — after about a year overseeing the federal agency.
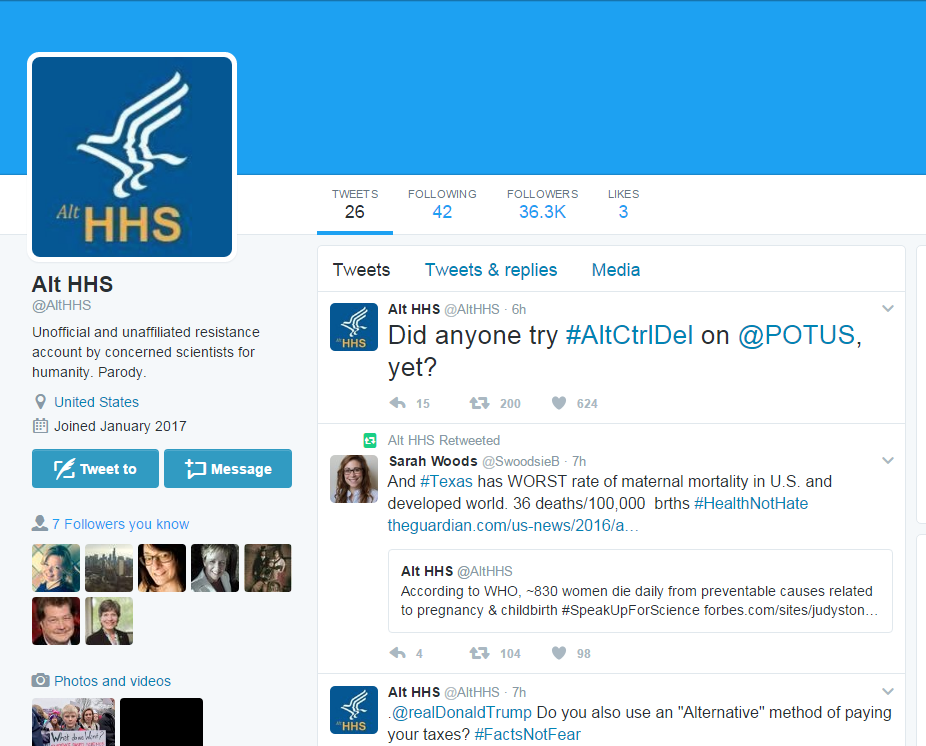
@AltHHS describes itself as "Unofficial and unaffiliated resistance account by concerned scientists for humanity."

A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.

A Kaiser Health News investigation shows that the system intended to help desperate patients is being manipulated by drugmakers to maximize profits and to protect niche markets for medicines already being taken by millions.

Several recent developments are of note as consumer electronics companies, hearing aid manufacturers, audiologists, physicians, consumer advocates and regulators prepare for a surge of new hearing devices and changes in the hearing healthcare system.
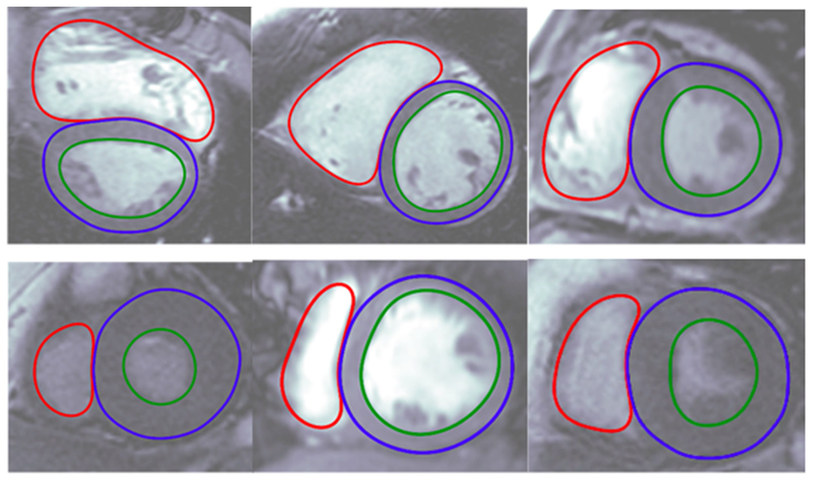
Arterys Inc.’s Cardio DL program applies artificial intelligence to automate tasks that radiologists have been performing manually.
Price has been a go-to congressman, a review of his records show, for medical special interests hotly sparring with regulators or facing budget cuts.

Munck Wilson Mandala Partner Greg Howison shared his perspective on some of the legal ramifications around AI, IP, connected devices and the data they generate, in response to emailed questions.

Several technology companies have developed ways to protect the pharmaceutical supply chain and support track and trace technology so counterfeit drugs can be easily spotted and identified.
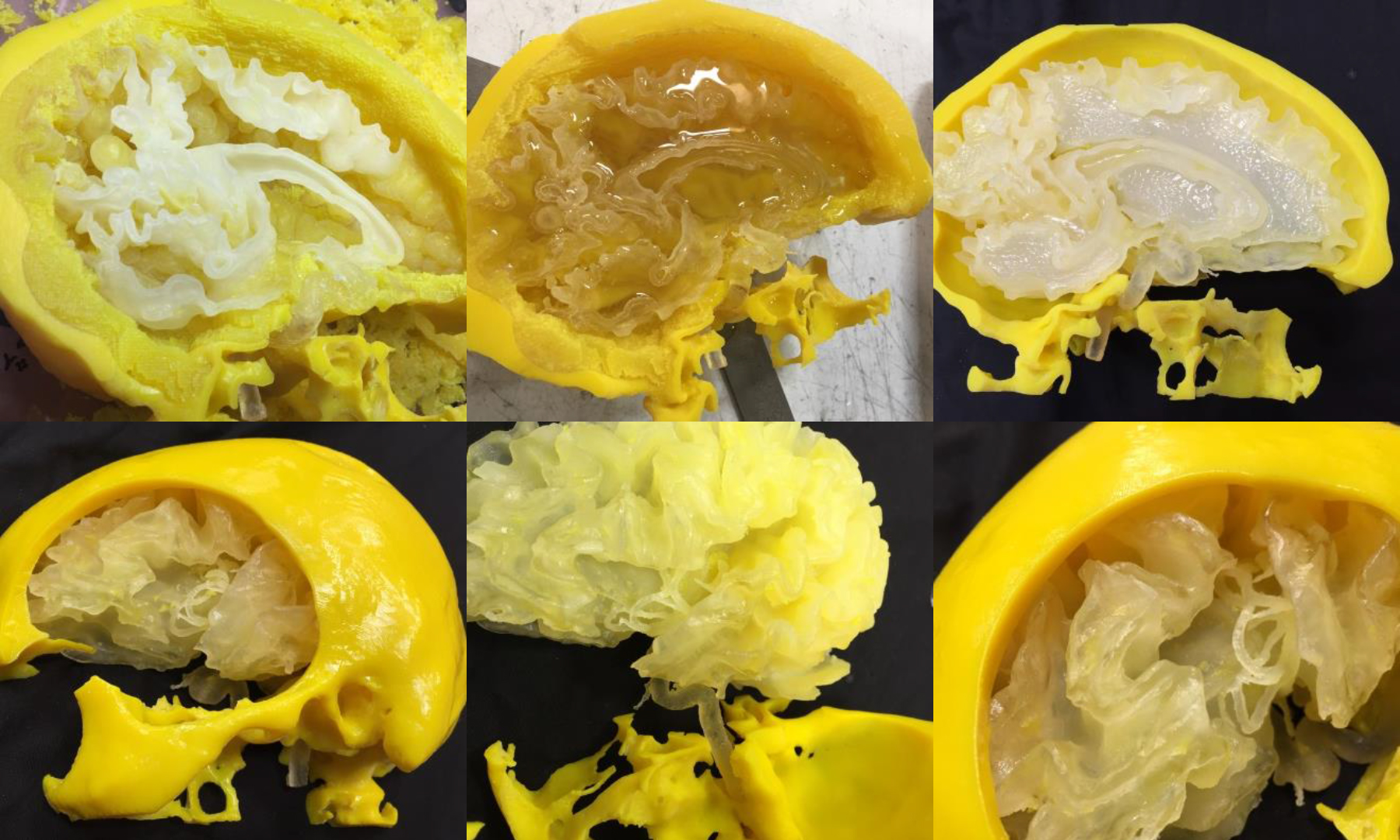
Analysts have predicted that 3D printing will be a major disruptive force in the healthcare industry before the end of the decade.

Cleveland Clinic CEO Dr. Toby Cosgrove will be one of 16 business leaders on President-elect Donald Trump's President's Strategic and Policy Forum, while Zenefit's David Sacks may join Trump's transition team, plus Apple loses a top health exec.

At least 79 cases of infection tied to Sorin heater-cooler units in the U.S. and worldwide have been reported to the FDA since 2010, including 12 deaths. Those numbers are expected to rise.

Here are some of the major health issues that are certain to come up in 2017.