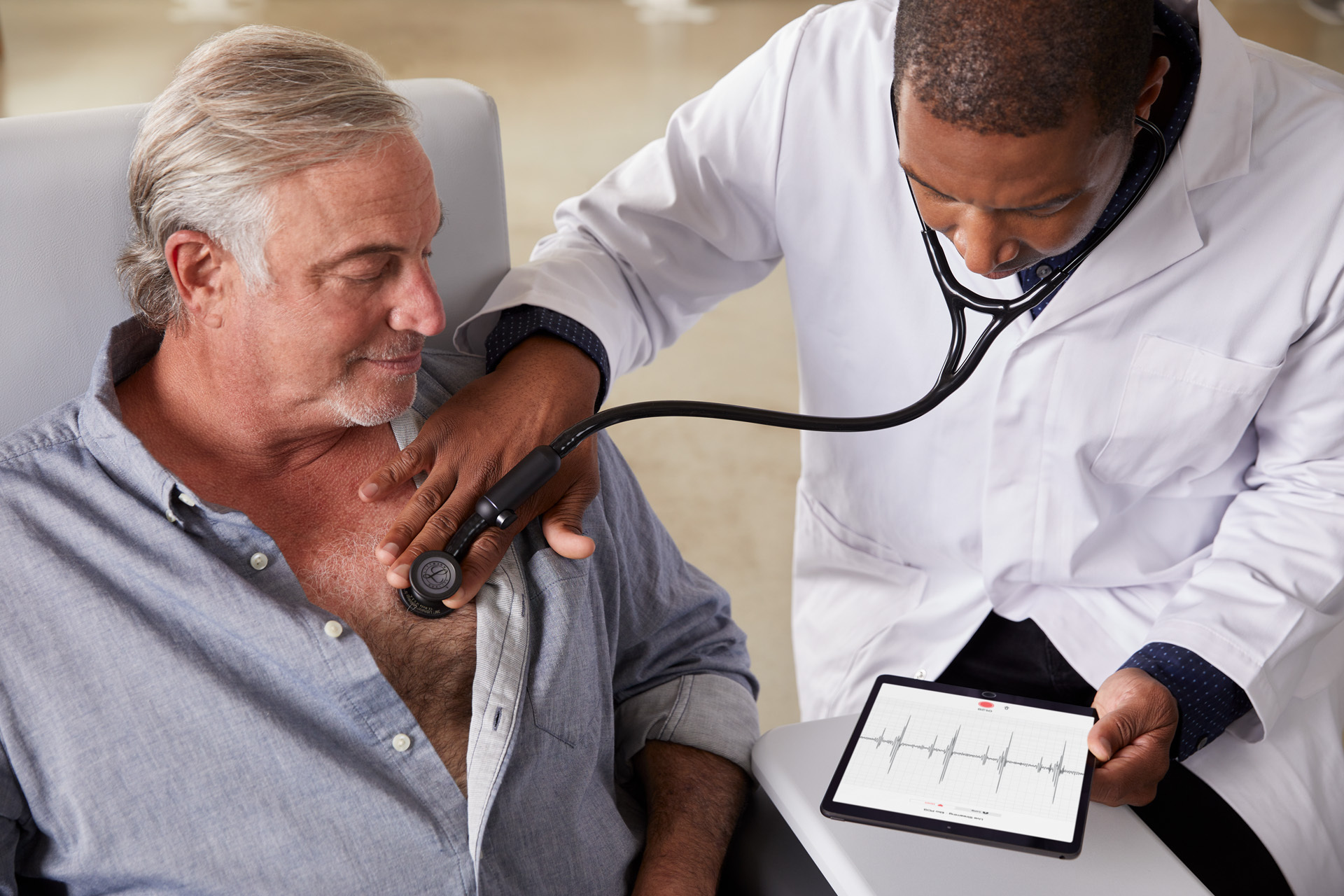
Eko partnered with 3M last month to develop a new digital Littman stethoscope. Photo credit: Mitch Tobias, courtesy of Eko.
After receiving FDA clearance to use its digital stethoscope to detect heart rhythm abnormalities, startup Eko plans to build a suite of telehealth tools around its connected devices. The Berkeley, Calif.-based company, which designed a combination stethoscope and electrocardiogram (ECG), raised a $65 million series C round.
Eko plans to use the funds to expand its new telehealth platform and algorithm to more clinics, and launch an at-home monitoring program for cardiopulmonary patients.
“The explosion in demand for virtual cardiac and pulmonary care has driven Eko’s rapid expansion at thousands of hospitals and healthcare facilities, and we are excited for how this funding will accelerate the growth of our cardiopulmonary platform,” Eko CEO and Co-Founder Connor Landgraf said in a news release.
Eko has been selling its digital stethoscope since 2015, but it got clearance for an algorithm to detect heart murmur and atrial fibrillation in January.
Wearable device makers, such as Apple Watch and Fitbit, have also gotten clearance for tools to detect atrial fibrillation. But while these devices are focused on alerting consumers to a potential irregularity, Eko intends for its systems to be used by clinicians to help them detect and diagnose irregular heartbeats. And while smartwatches also use ECG sensors to track electrical signals, Eko’s stethoscope feature lets it capture the heart’s sound, which could be useful for detecting heart murmurs.
More recently, Eko has struck collaborations centered on this new feature. The company launched a new line of digital stethoscopes with 3M earlier this year, and also struck a partnership with AstraZeneca to develop digital health tools to screen for cardiovascular diseases, including heart failure.

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
Highland Capital Partners and Questa Capital led Eko’s recent funding round. Corey Mulloy, a partner at Highland, and Ryan Drant, Questa’s founder and managing partner, will also join the company’s board of directors.
“The massive market need for telehealth is not going away,” Rob Toews, a principal at Highland Capital Partners, said in a news release. “Regulatory and reimbursement changes have been underway to support this growth. Eko is uniquely positioned in this space because their technology addresses crucial clinical needs that other companies cannot satisfy, and Eko’s platform is very easy to deploy and scale. We are excited to partner with Eko in this next phase of their growth.”












