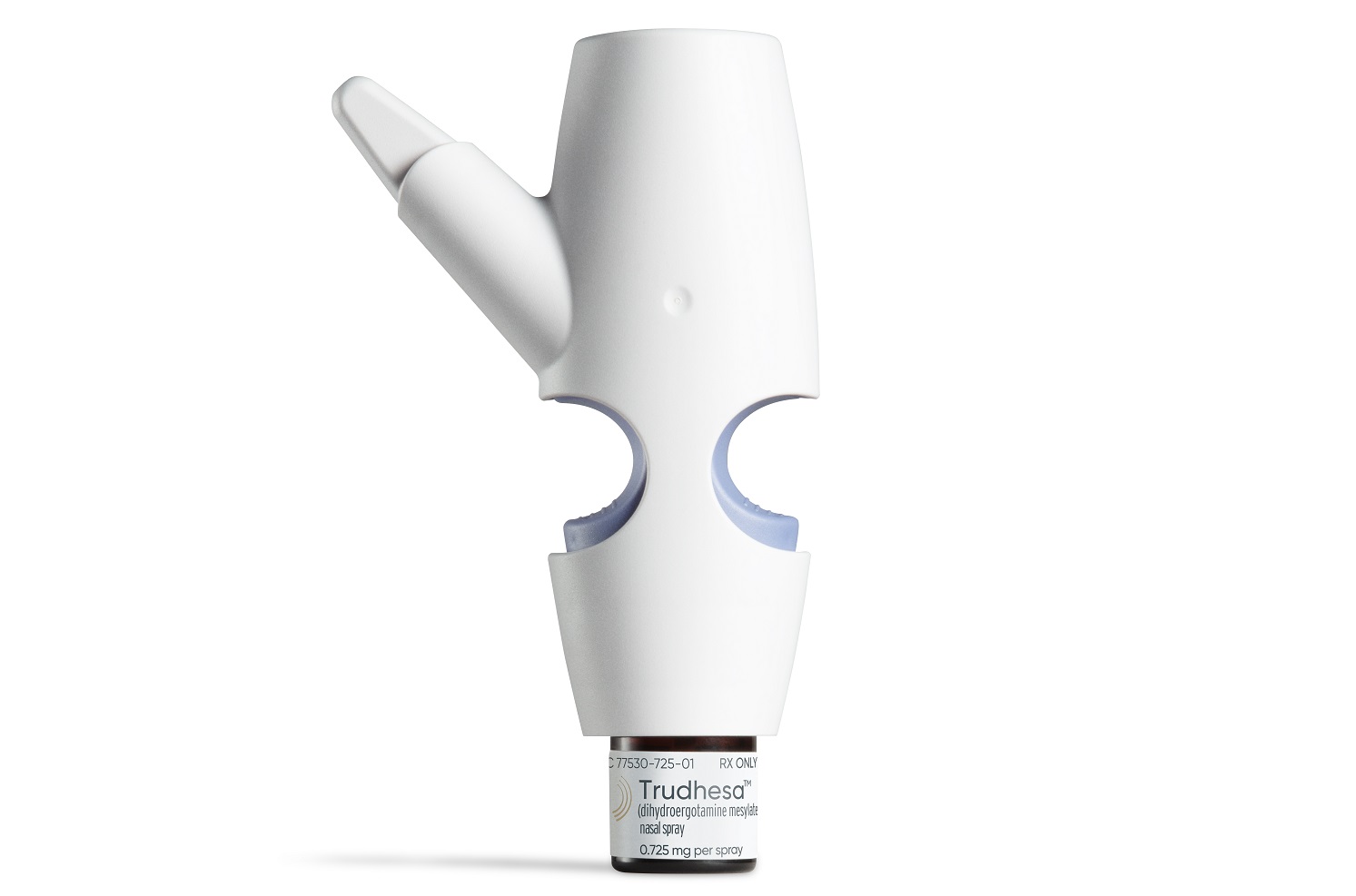
Impel NeuroPharma’s new take on delivering an old migraine drug won FDA approval Friday, giving patients a faster-acting way to respond to debilitating migraine attacks.

Behavioral Health, Interoperability and eConsent: Meeting the Demands of CMS Final Rule Compliance
In a webinar on April 16 at 1pm ET, Aneesh Chopra will moderate a discussion with executives from DocuSign, Velatura, and behavioral health providers on eConsent, health information exchange and compliance with the CMS Final Rule on interoperability.
The Impel product, Trudhesa, is a nasal spray formulation of the migraine drug dihydroergotamine mesylate (DHE). The regulatory decision covers acute treatment of migraine, with or without aura, in adults.
DHE has been used as a migraine treatment for decades, dosed as injections, infusions, or nasal sprays. But injections and infusions must be given by a physician at a medical site, and while patients can give administer nasal sprays themselves, these doses may not deliver enough of the drug. The triptans drug class comprise the most commonly prescribed drugs for treatment of acute migraine, and they offer patients a pill option. But Impel notes that studies have shown that these drugs must be taken early on in the migraine attack, and it can take hours before a patient feels their effect.
Impel’s proprietary Precision Olfactory Delivery (POD) technology is designed to deliver DHE to the upper nasal cavity, a region rich in blood vessels that can rapidly absorb the drug. This approach is meant to quickly get the drug into the bloodstream without subjecting patients to a needle. Furthermore, the drug can be taken at any time during a migraine attack
The FDA’s decision was based on the results of an open-label Phase 3 study enrolling 360 people who suffer at least two migraine attacks per month. Patients were instructed how to self-administer the drug and record their migraines in a diary. The study evaluated patients for 24 weeks; a subset of participants were followed for an additional 28 weeks. The main goal was to measure the number of serious adverse events over the course of the study.

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
Across both groups in the clinical trial, Impel said more than 5,650 migraine attacks were treated. Impel reported seven serious adverse events in five patients (1.4%) over the course of 52 weeks. None of those events were nasal related and none were deemed to be related by the clinical trial investigator to be due to Trudhesa. The adverse events that were related to the treatment were characterized as mild and transient, and included nasal congestion, nausea, nasal discomfort, and an unpleasant taste.
Full results from the pivotal study were published last month in the scientific journal Headache. Impel started a 12-month extension study to gather more data on long-term use of the drug, but that study was ultimately deemed unnecessary due to the lack of safety problems observed in the six-month group.
Despite the lack of safety signals observed in clinical trials, Trudhesa’s label carries a boxed warning that alerts physicians and patients that the drug should not be taken with medications (or food, such as grapefruit) containing CYP3A4 inhibitors. A dangerous drug interaction can elevate DHE levels in the blood, potentially leading to a narrowing or obstruction of peripheral arteries, which in turn limits the distribution of blood to the limbs. The warning is similar to warnings on labels of other DHE products.
In securing FDA approval for Trudhesa, Impel will reach the market ahead of Satsuma Pharmaceutical. That San Francisco-based company is developing a drug-device combination product that also administers DHE. But Satsuma hit a setback after its treatment failed in Phase 3 testing last year. After analyzing the clinical trial data, Satsuma made small modifications to its device and drew up plans for a new pivotal study, which is currently underway.
Impel went public in April, raising $80 million as the company prepared for commercialization of its first POD product in migraine. The company’s pipeline includes POD product candidates for autism spectrum disorder and Parkinson’s disease.
Impel expects to launch Trudhesa next month. The company plans to discuss Trudhesa’s approval during a conference call scheduled for 8:30 am Eastern on Sept. 7.
Photo by Impel Neuropharma












