The very common Peripheral Blood Smear review, used time and again for diagnostics, is typically conducted by a human analyzing the sample on a glass slide under a microscope and checking cell types, scouring for abnormalities that could indicate cancer or other diseases. The process is often not only tedious and laborious, but can be quite time consuming, requiring specific training and expertise to do so well, according to Itai Hayut, co-founder and CEO of Scopio Labs, which raised $50 million on Wednesday.
Further, traditional methods of manual microscopy force the technician to choose either a high resolution or a large field view, as improved resolution comes at the cost of the field of view. The method of digitizing snapshots of a few cells, an improvement over the manual method, still poses its own slew of challenges as doing so removes the cells from their full context, and as a result, requires the slide be kept on hand.
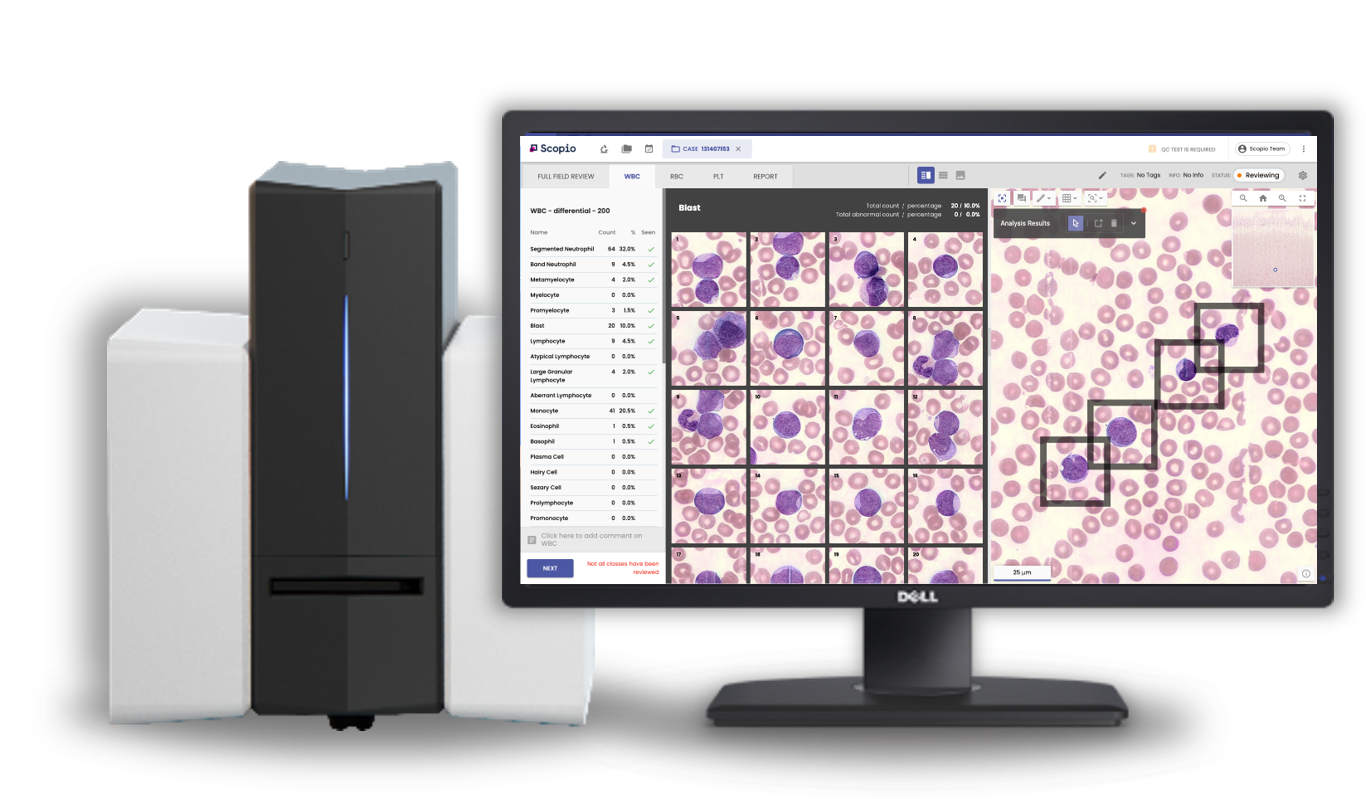
Enter Scopio Labs. Its FDA-cleared imaging and AI analysis platform not only enhances diagnostic capabilities by allowing for both high resolution and large field view, but also enables remote work, claims Hayut. Scopio’s end-to-end workflow technology both digitizes and analyzes blood samples. Its device employs computational photography to create ultra-high-resoloution images that include the entire sample slides. The slide can be zoomed at 100X magnification to see individual cells as well as show the sample in context. Further, Scopio’s product allows hematopathologists and clinicians to access the images from anywhere, not requiring them to be in the lab or at the microscope.
The other key distinction of its product includes the AI-based decision-support solution that Scopio hopes will analyze the samples by helping with pre-classification, detection, and quantification for blood cells. Scopio’s Full-Field Peripheral Blood Smear (Full-Field PBS) Application is FDA-cleared and CE-marked.
All these capabilities swayed investors who poured in $50 million in a Series C funding round into Scopio base in Tel Aviv, Israel. announced Investors included OurCrowd, Ilex Medical, Aurum Ventures, Mizrahi Tefahot Bank Invest, Olive Tree Ventures, LR Group, Mivtach Shamir, angel investors, and a strategic investor from the company’s field of operation. To date, Scopio has raised $84 million.
“Existing digital cell morphology platforms are in fact only semi-digital solutions. They only provide snapshots of single cells without a scan of the slide. This limited amount of information forces labs to still keep the physical slide and microscope on hand as in many cases the context of the slide is critical for making the right clinical decision,” contends Hayut in an email. “Without full-field capabilities, no competitor can offer a true remote solution. With Scopio, offsite hematopathologists and clinicians have the same browser-based access to the full-field digital image, AI results, annotations, and insights as anyone physically in the lab.”

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.

Scopio envisions its field imaging technology allowing the field to not revert back to a manual microscope methods, according to Hayut. The hope is that the tech will enable an end-to-end digital workflow and offer true remote hematology, which Hayut dubs “the field of tele-hematology.”
In the last decade, remote diagnostic healthcare has increased, such as telereadiology. Further, the pandemic additionally prompted the push for remote options. Scopio sees its tech as advancing telehematology, according to the press release.
Hayut added that he envisions Scopio’s products enabling both early diagnosis as well as disease monitoring via imaging and analyzing a vast number of cells at a high resolution. He describes it as orders of magnitude above what a person or previous digital tech could handle.
When asked how the company will utilize the series C funding, Hayut had several goals.
“We are expanding our commercial operations globally, scaling up manufacturing and continuing development of the company’s portfolio to fulfill Scopio’s vision of large-scale morphological analysis in hematology (including bringing the world’s first Bone Marrow Aspirate application to market in 2022),” Hayut said. “Scopio will also be doubling the current number of employees in the coming year.”
Photo: David Garb09














