Trial Data Looks Promising for ‘IUD For Men’
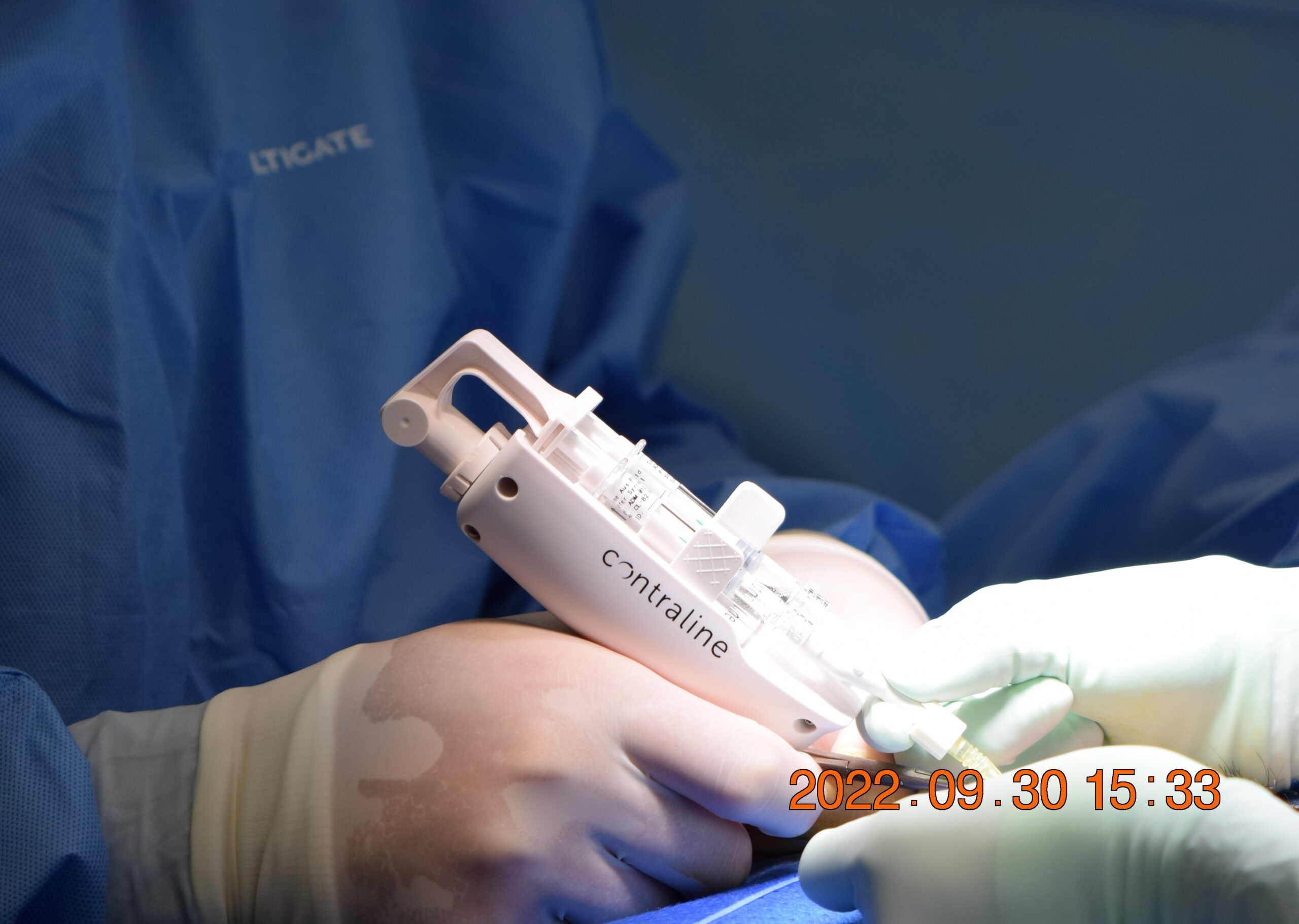
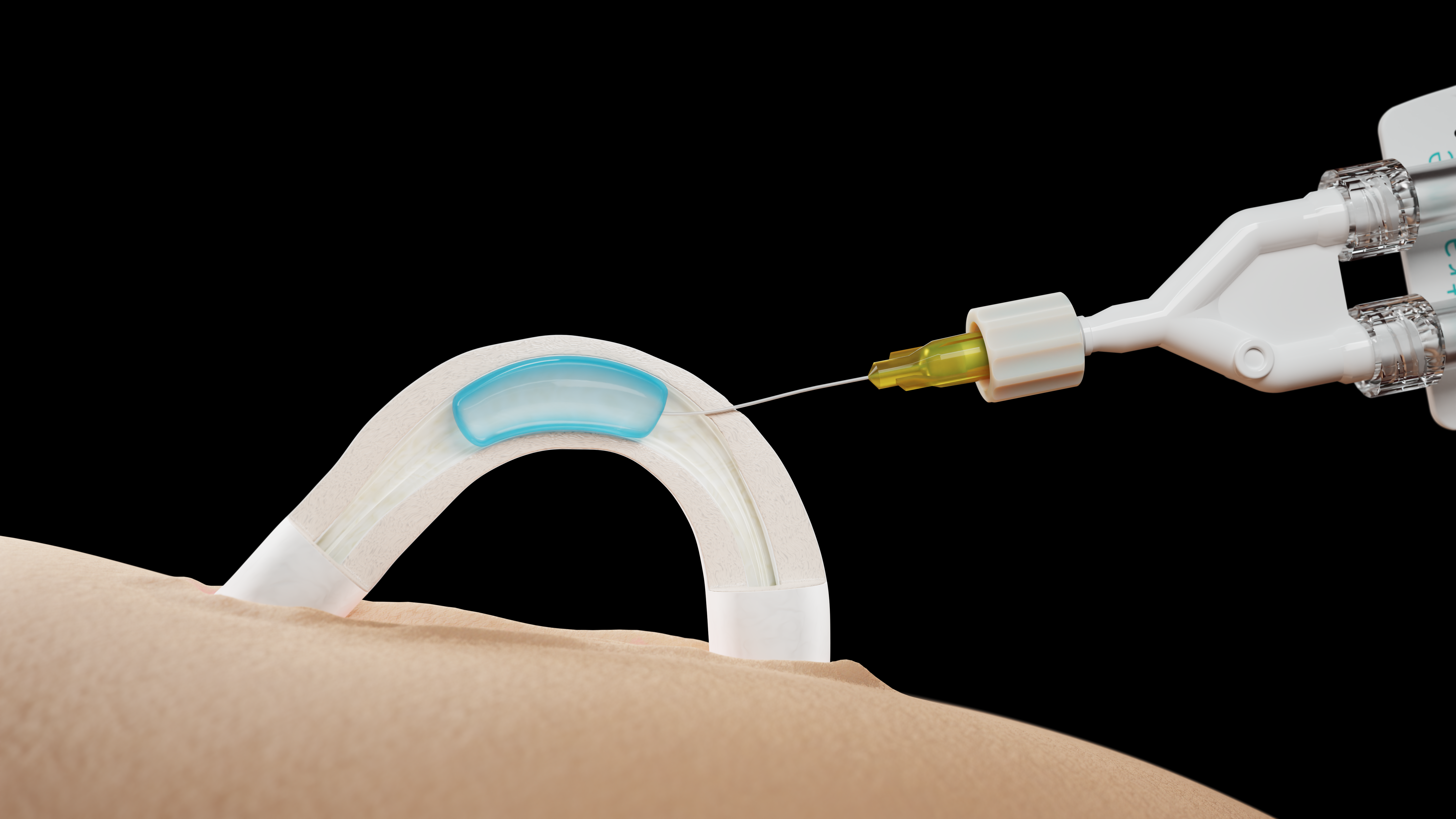
Contraline released promising data from the clinical trial it’s conducting to test the efficacy of Adam, its male birth control product. The nonhormonal gel seems to be doing a good job of blocking the flow of sperm to the vas deferens, and no serious adverse events have been reported.