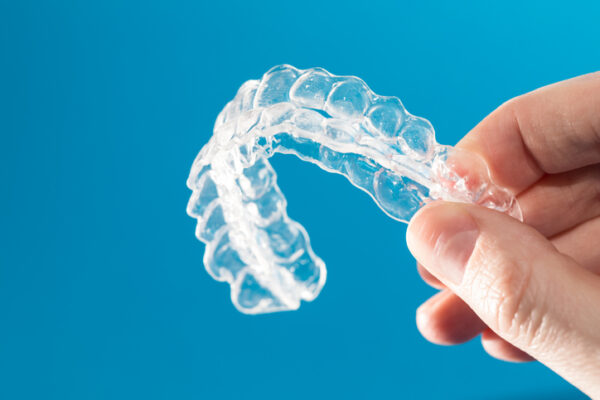
A startup that lets orthodontists design and manufacture aligners in-office closed an $18 million funding round on Monday. Redwood City, California-based uLab systems developed a software that allows orthodontists to quickly develop aligners for patients and manufacture them using a 3-D printer.
Unlike the recent spate of dental startups, uLab Systems doesn’t send aligners directly to patients. Rather, it leaves it up to orthodontists to come up with treatment plans during an office visit.

Transforming Clinical Content with Ambient & Generative AI
Sheila Bond, MD, talked about the latest trends regarding integration of AI in healthcare.
Hedge fund Park West Asset Management led the funding round, along with support from minority investors, including Ortho Studio Express.
“We are excited about what lies ahead for uLab as we advance the aligner industry to provide better solutions for the orthodontist and the patient,” uLab CTO Charlie Wen said in a news release. “Importantly, we are proud to have a partner in Park West as we work to give control of the treatment plan back to where it belongs – the orthodontist.”
The company claims orthodontists can make treatment plans in as little as 10 minutes using its software. Those that have a 3-D printer in their office can also print out the aligners, allowing patients to get them in the same day as their appointment.
uLab Systems seems to be poising itself as a convenient in-office alternative to direct-to-consumer aligner companies, which have come under fire recently as orthodontists and dental groups argue they can’t provide adequate consultation to patients. In September, orthodontists and a group of patients filed a class action lawsuit against SmileDirectClub. 21 of the plaintiffs withdrew their claims three months later.
uLab, which was founded in 2015, currently has 250 orthodontists using its systems. As of April, the company said 13,000 cases had been planned on its software.
The company received 510(k) clearance from the FDA for its software in 2018.
Photo credit: scyther5, Getty Images








