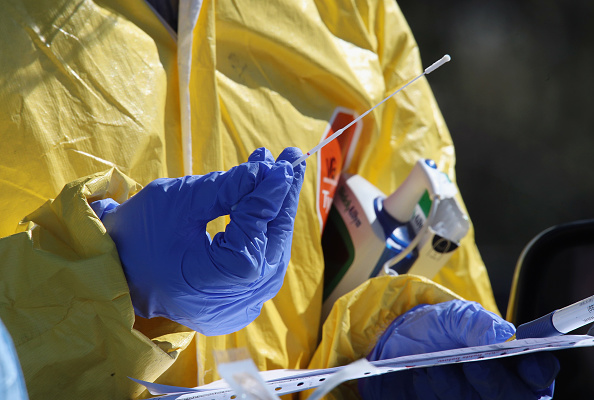
A close-up view of a swab used by medical workers tend to administer the coronavirus test at the drive-in center at ProHealth Care on March 21, in Jericho, New York.
Patients can now swab themselves for Covid-19 tests, the Food and Drug Administration announced this week in an updated guidance. But the agency still isn’t allowing at-home tests.
Prior to this week, healthcare providers were required to swab deep inside a patient’s nasal cavity, a somewhat uncomfortable process. But for patients that already have Covid-19 symptoms, the FDA now says that swabbing the front of the nose is acceptable.
Vice President Mike Pence announced the shift in a press conference on Thursday, saying the change had already begun across the country.
“It allows for self-collection and it also relieves the burden on healthcare protection. It saves personal protective equipment from being expended when people can administer the tests themselves,” he said.
The FDA’s new stance follows a study conducted by UnitedHealth Group that showed for 500 patients at OptumCare facilities in Washington, self-administered swab tests accurately detected Covid-19 in more than 90% of positive patients. UnitedHealth Group shared the results of the study on Wednesday, which the insurer said it planned to publish in a peer-reviewed medical journal.
“We know that broad, rapid and accurate testing is essential to addressing the COVID-19 crisis, yet the current clinician-administered process significantly limits testing capacity, puts frontline health care workers at risk of COVID-19 exposure, and is unpleasant for patients,” Dr. Yuan-Po Tu said in a news release. He is the study lead and an infectious disease expert at The Everett Clinic, which is part of Optum Health. “Making simple, patient-administered testing widely available will substantially improve testing efficiency, while protecting health care workers and preserving urgently needed personal protective equipment, such as face masks, gowns and gloves.”

As Healthcare and Biopharma Companies Embrace AI, Insurance Underwriters See Risks and Opportunities
In an interview, Munich Re Specialty Senior Vice President Jim Craig talked about the risk that accompanies innovation and the important role that insurers play.
According to the FDA’s updated guidance, getting a nasopharyngeal specimen from further back in the nasal cavity is still the preferred choice. The simpler, self-conducted swab tests could only be used by symptomatic patients at collection sites.
Even with these changes, the FDA still has not yet approved any at-home Covid-19 tests. After a number of startups rushed to put together at-home testing kits last week, the agency specified on Friday that at-home testing is not allowed, leaving the companies on pause.
“Due to concerns with specimen stability, transport, and appropriate collection materials, self-collection at home or at sites other than designated collection sites staffed by (healthcare providers) is currently not recommended,” the FDA stated in its updated guidance.
But for those startups, there still may be a path forward. Everlywell, a direct-to-consumer lab testing startup, said it had been meeting regularly with the FDA since it made its statement last Friday.
“The test will likely use a short nasal swab for easy collection and will include free telehealth consultations with an independent physician for those with positive results to receive diagnosis at home,” the company wrote in an emailed statement. “We hope to make it available to the public soon.”
This story has been updated with comments from Everlywell.
Photo Credit: Bruce Bennett, Getty Images








