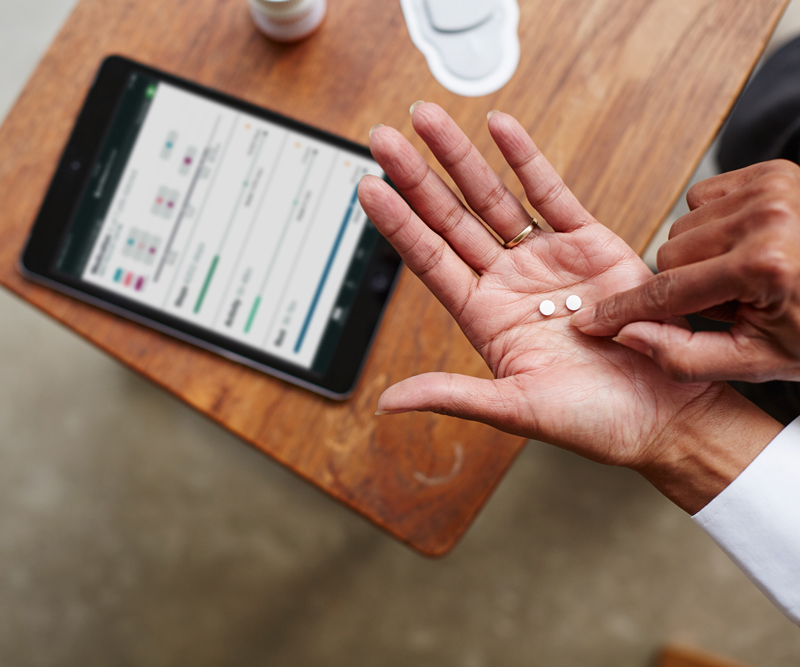
Proteus’ solution allows physicians to track medication adherence by taking a pill with a tiny sensor. The patient also wears a patch, which transmits that information to a tablet. Source: Proteus digital health
Three years ago, the FDA approved the first medication with an embedded sensor. The developer of these sensors, Proteus Digital Health, touted them as a regulatory breakthrough, with the ambitious goal of putting its sensors in more approved medications. It struck a partnership with Otsuka Pharmaceutical, and eager investors put more than $500 million into the company.

The Power of One: Redefining Healthcare with an AI-Driven Unified Platform
In a landscape where complexity has long been the norm, the power of one lies not just in unification, but in intelligence and automation.
Now, the Redwood City-based digital medicines startup has filed for bankruptcy. Otsuka — and reportedly others — are looking to purchase Proteus’ technology. In an emailed statement, Proteus said its programs would remain in place and that it does not expect any layoffs as a result of the bankruptcy filing. The company currently has 93 employees, down from roughly 300 last year.
“Filing for bankruptcy protection allows Proteus to continue its sales process in a more concerted and efficient manner while also continuing to run the business as usual,” the company stated.
Lawrence Perkins of SierraConstellation Partners has been named the company’s interim CEO, after serving as chief restructuring officer.
From a vaunted healthcare unicorn to a petitioner for Chapter 11 bankruptcy, how did the company falter? Experts said the company struggled to gain traction with insurers and patients.
In December, CNBC reported that Proteus was short on cash, after failing to close an expected $100 million funding round. The company reportedly furloughed its employees for two weeks in November.
Just a month later, the company saw its licensing agreement with Otsuka unravel, as first reported by Stat News. The two had worked together to develop Abilify MyCite, an antipsychotic medication with an embedded sensor. Otsuka confirmed that it had acquired the license to develop mental health treatments using Proteus’ technology, and would no longer pay royalties going forward.
Otsuka said it did not expect the bankruptcy to affect its ability to deliver Abilify MyCite.
“We want to make it clear that this development is not expected to have an impact on our digital medicine program,” the company wrote in an emailed statement.
Proteus saw early success because medication adherence has been a stumbling block for both pharmaceutical companies and insurers. The majority of patients don’t take their pills as prescribed, costing an estimated $100 billion to $300 billion per year.
“It sounds beautiful: ‘This is going to change medicine as we know it. We finally have a digital sensor.’ Then you start to dig into the details. It’s always, the devil’s in the details,” said Vasudev Bailey, a partner at ARTIS Ventures.
Worth the cost?
Proteus’ solution required patients to wear a patch on their body with a digital receiver that would then detect if they had taken the pill. The cost was also a barrier — Abilify MyCite reportedly cost $1,650, at least double the cost of the brand name drug and several times the cost of the generic.
“Do we really see our parents, our grandparents, ourselves, wanting to do that?” Bailey said. “I feel like this was the orchestration of an idea that seems to fix the problem, but doesn’t pay much attention to the patient experience and what patients really want.”
Dr. James Levenson, a psychiatry professor for Virginia Commonwealth University School of Medicine, said there are simpler and cheaper ways to assess adherence, such as seeing if a prescription is being filled regularly, or even counting the number of pills in the bottle at an appointment.
The medication itself also may not have been a good fit for the sensor. Some patients with schizophrenia experience fears that they are being controlled or monitored by others.
“As a psychiatrist, I would still feel uneasy about prescribing an antipsychotic drug with that feature,” he wrote in an email.
Proteus also faced outstanding questions about how its treatment would create value for health systems, payers and patients. The company needed to provide health economics and outcomes research to prove that point. For example, a paper published last year in the British Medical Journal raised concerns that there was no evidence that the drug produced better adherence.
“That’s where the narrative starts to slowly unravel. It’s really challenging to do that. It’s really challenging to get consumers to even want this,” Bailey said. “Why would an insurance company pay more for you to take it? I don’t think they closed the gaps of answering all of these questions.”
The sheer amount of capital raised by Proteus may also have put pressure on the company to reach certain milestones more quickly, giving it less flexibility to iterate on its product and figure out the best fit. The terms of the company’s later funding rounds became very expensive, with participating preferred stock, said Michael Greeley, co-founder of Flare Capital Partners.
“They’re exciting, they get a lot of headlines, but you have to be able to support that valuation if you need to raise more capital,” he said. “That’s where it can spiral against you very quickly.”
A second life for sensors
Still, Proteus’ technology may see a second life as other companies look to buy it. Even if Otsuka has expressed interest, that doesn’t necessarily mean the IP will immediately fall to them, Greeley said. They will likely face many bidders over a long bankruptcy process.
“I think someone will try to push this forward,” he said. “I think it’s a setback for the category.”
While Proteus’ technology is not necessarily a digital therapeutic given it mainly monitored adherence, digital and pharma collaborations have been rocky lately.
Meanwhile, emerging competitors, such as etectRx, may also face a challenge as potential investors and other stakeholders become a little more wary. EtectRx received FDA clearance for its ‘smart pill’ last year; its system uses a wearable lanyard instead of a patch.
“It’s going to be a hard road ahead,” Bailey said. “I think this (technology) will find a home to live in and people will still advance this. So much work and effort goes into developing these things. You still want someone’s life to get better.”








