Bristol Myers Squibb cancer immunotherapy Opdivo has become a standard treatment for melanoma but the pharmaceutical giant has been testing that blockbuster drug in combination with other therapies hoping to further improve patient outcomes. The company is now reporting late-stage clinical data showing that a pairing with one of its experimental immunotherapies works better at stopping the cancer from progressing.

Tackling Rising Drug Costs and Growing Popularity of GLP-1’s
See how Quantum Health is providing the steps to help their members tackle the cost of specialty medications and other drugs.
BMS tested its experimental drug, relatlimab, combined with Opdivo in a Phase 3 study that compared the combination treatment against Opdivo alone in patients whose melanoma had spread or could not be removed by surgery. Median progression-free survival, a measure of how long a patient lives before the cancer progresses, was 10.12 months for the combination therapy. For patients treated with Opdivo alone, progression-free survival was a median 4.63 months.
BMS reported preliminary data for the Phase 3 study in March. More detailed results were disclosed for the first time in an abstract released Wednesday ahead of the annual conference of the American Society of Clinical Oncology. The data are scheduled to be presented at a June 6 session during the conference.
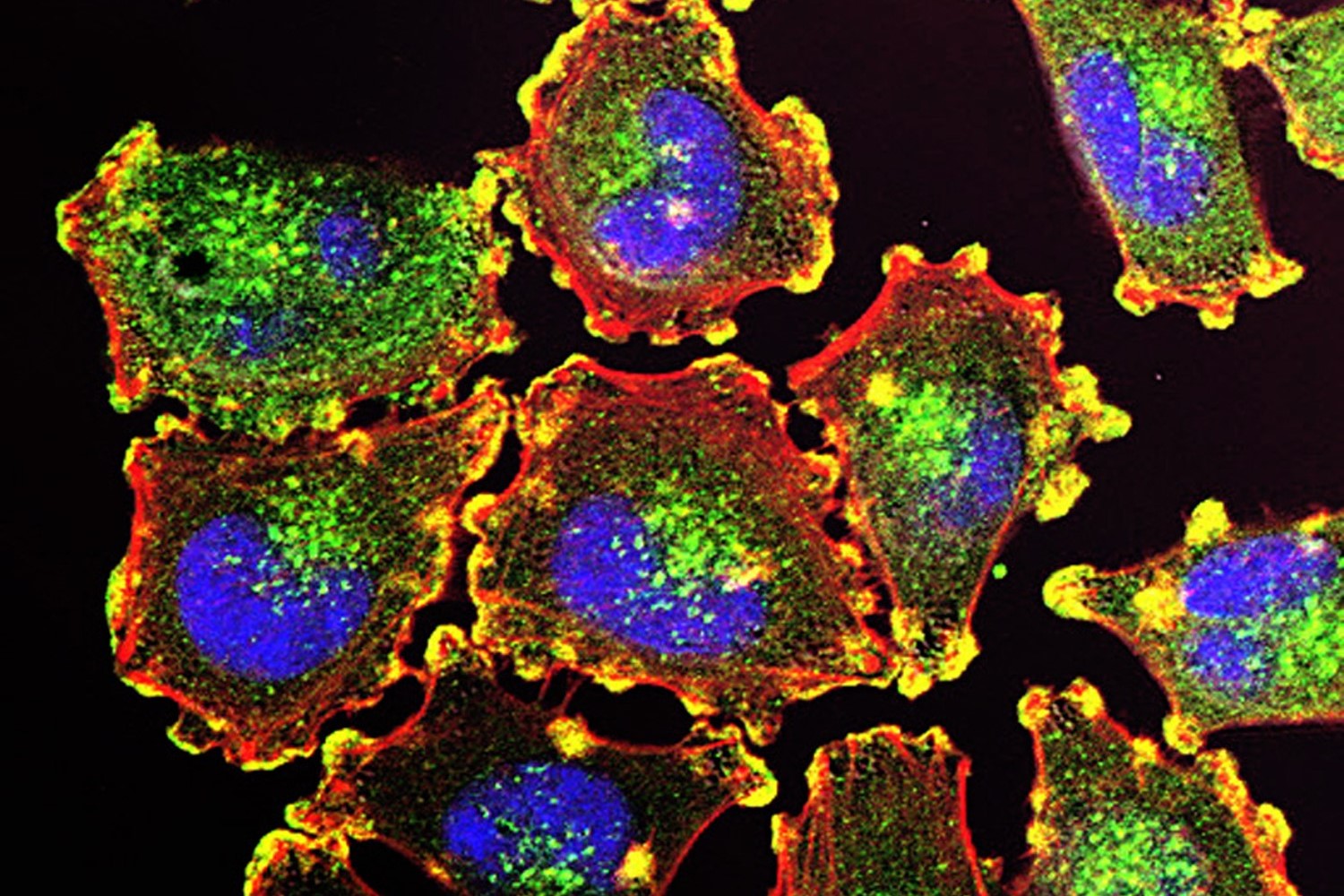
Opdivo is a type of immunotherapy called a checkpoint inhibitor. It targets PD-1, a checkpoint protein that stops immune cells from recognizing tumors. Blocking this protein enables immune cells to recognize and go after cancer cells. Relatlimab is also a checkpoint inhibitor, but it targets a different checkpoint protein called lymphocyte-activation gene 3 (LAG-3). BMS said that by blocking both checkpoint proteins, the combination may offer a stronger immune response against cancer.
The drug combination was tested in a global, double-blind Phase 2/3 clinical trial enrolling 714 patients. Those patients were randomly assigned to receive an intravenous infusion of the combination treatment or Opdivo every four weeks until the disease recurred, drug toxicity became unacceptable, or the patient declined to continue participating in the clinical trial. The study’s main goal was to measure progression-free survival; secondary endpoints were overall survival and objective response rate. BMS said that follow-up for the secondary endpoints is ongoing and the company remains blinded to the study.

Understanding EGPA: The Role of Eosinophils and Advancements in Treatment Options
FASENRA® (benralizumab) injection, for subcutaneous use, 30 mg is indicated for the treatment of adult patients with eosinophilic granulomatosis with polyangiitis (EGPA). FASENRA provides a treatment option for HCPs to consider when managing this challenging disease.
The drug combination was well tolerated by patients and no new safety signals were reported in the study. But adverse events categorized as Grade 3 or 4 were reported in 18.9% of patients in the combination arm compared to 9.7% of patients in the Opdivo arm, BMS reported. More patients in the combination arm discontinued treatment due to those adverse events, 14.6% compared with 6.7% in the Opdivo arm.
BMS isn’t the only company developing an antibody that blocks LAG-3. Merck is testing its LAG-3 inhibitor, favezelimab, in combination with its blockbuster immunotherapy Keytruda, in colorectal cancer. On Wednesday, the company released an abstract detailing Phase 1 results.
Relatlimab is the third checkpoint inhibitor developed by BMS. In addition to Opdivo, the company’s portfolio also has Yervoy, a checkpoint-blocking antibody that targets a protein called CTLA-4.
In a prepared statement, Jonathan Cheng, senior vice president and head of oncology development at BMS, said the results from the relatlimab study provide evidence that a “dual immunotherapy” approach may help more patients and the company plans to bring the data to regulators.
“While there have been significant treatment advances and long-term survival benefits provided by checkpoint inhibitors over the years, there remain patients with metastatic melanoma who could benefit from another innovative approach,” Cheng said. “We look forward to discussing these registrational data with health authorities to potentially bring this treatment to patients.”
Public domain image by Julio C. Valencia via the National Cancer Institute






