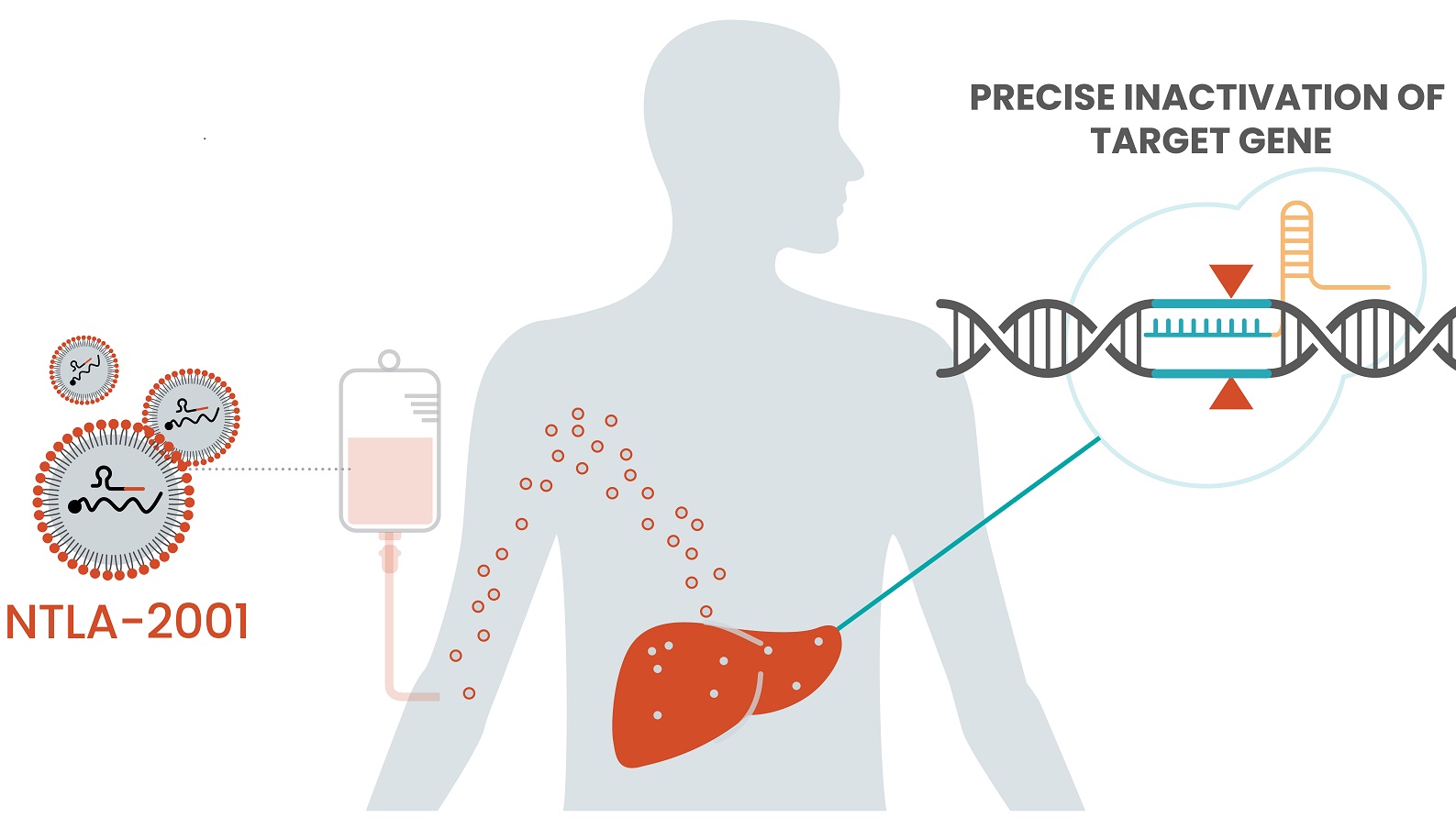
An Intellia Therapeutics gene-editing therapy that is infused into patients and circulates throughout the body now has early data from a small clinical trial showing it can do its editing work inside patients safely and effectively, bringing validation to the company and its technology, and to the field of gene editing broadly.
The experimental therapy, NTLA-2001, is in development as a treatment for transthyretin amyloidosis, or ATTR, a rare, inherited disorder that causes the transthyretin protein to misfold. This misfolded protein builds up in the body, particularly nerve tissues and the heart, and becomes fatal. Intellia’s therapy is designed to use CRISPR to inactivate the gene that causes ATTR, potentially offering a cure.
The Phase 1 study, which is being conducted in the U.K. and New Zealand, has preliminary results for two doses in six patients, three patients for each dose. In the lower dose group, blood levels of TTR were reduced by an average of 52% at day 28. In the higher dose, the average reduction was 87%, which included one patient with a 96% reduction. The study also demonstrated safety with no evidence of adverse events or liver toxicity.
Cambridge, Massachusetts-based Intellia presented the preliminary Phase 1 results at the annual meeting of the Peripheral Nerve Society on Saturday. They were concurrently published in the New England Journal of Medicine.
“This is the first demonstration of CRISPR-based in vivo gene editing in humans,” said Julian Gilmore, a professor of medicine at University College London and the Phase 1 study’s national coordinating investigator, speaking during an Intellia conference call Monday. “These findings provide proof of concept for a promising new therapeutic strategy.”
ATTR is hereditary, caused by mutations to the TTR gene. The disease affects an estimated 50,000 people worldwide, Gilmore said. In some cases, ATTR is acquired, though the prevalence of this “wild type” is unknown. Wild-type ATTR leads primarily to cardiomyopathy. The Intellia study in hereditary ATTR is continuing, and higher doses will be tested with the goal of achieving even greater reduction of TTR. A higher dose is expected lead to an improvement in symptoms, mobility, and fewer hospitalizations for heart failure, Gilmore said.
Intellia has developed a modular technology platform that supports both in vivo and ex vivo gene therapies. The in vivo gene-editing therapies research is focused on diseases caused by genes in the liver. Unlike some genomic medicines that are delivered by engineered viruses, Intellia’s in vivo therapies reach their targets via a lipid nanoparticle. That distinction is notable because a virus can cause the immune system to produce antibodies against it, rendering any additional doses ineffective. Intellia’s lipid nanoparticle (LNP) platform poses no such problem, CEO John Leonard said during the call.
“One of the reasons we’re excited about the LNP platform is that, in fact, you can redose,” he said. “This was a very important part of putting the modular system together, and the chemical-based approach as opposed to a virological delivery.”
Leonard said those that did not receive the maximum effective dose in the clinical trial would be able to come back and receive the higher dose. That treatment will be given outside of the clinical trial.
NTLA-2001 is also showing early signs of an efficacy advantage. The treatment options for ATTR include Alnylam Pharmaceuticals’ Onpattro, which in 2018 became the first FDA-approved product based on a mechanism called RNA interference. Such medicines stop a gene from producing a disease-causing protein and the drug has demonstrated 80% reduction in TTR. It’s too soon to know the durability of the Intellia therapy but the early data suggest that NTLA-2001 can reduce TTR levels to potentially curative levels. Furthermore, the Intellia drug is a one-time treatment compared to Onpattro, which must be infused every three weeks.
The NTLA-2001 Phase 1 study is designed to enroll four groups of patients. Enrollment of the third group is currently underway, but Leonard said that the company is not under any obligation to start the fourth group if the company can select a recommended dose from tests in three groups. Chief Medical Officer David Lebwohl said that once the company identifies that dose, the therapy will proceed to start the second part of the study, which will test the recommended dose in eight patients in a single group.
The ATTR data so far give Intellia additional confidence in NTLA-2002, a gene knockout therapy that is in development for another rare disorder, hereditary angioedema (HAE). Lebwohl said that the HAE therapeutic candidate comes from the same platform, so its safety is expected to be similar to the ATTR therapy. He said the company will discuss details about the clinical trial design for NTLA-2002 as the company gets closer to beginning the study.
In a research note, RBC Capital Markets analyst Luca Issi described the NTLA-2001 data as a “home run” for Intellia. Results from tests in a larger group of patients are neccessary, as well as longer follow-up of those patients, but “we think the data is a major de-risking event for the platform, and we think HAE (NTLA-2002) could be next,” Issi wrote.
Intellia is developing NTLA-2001 with Regeneron Pharmaceutical under a collaboration that began in 2016. The deal calls for the partners to develop CRISPR-based therapies that target the liver and to improve the Intellia genome editing technology platform. The initial collaboration has since been expanded to include hemophilia.
Intellia plans to present additional data from the NTAL-2001 study at a medical or scientific meeting later this year.
Graphic by Intellia Therapeutics










