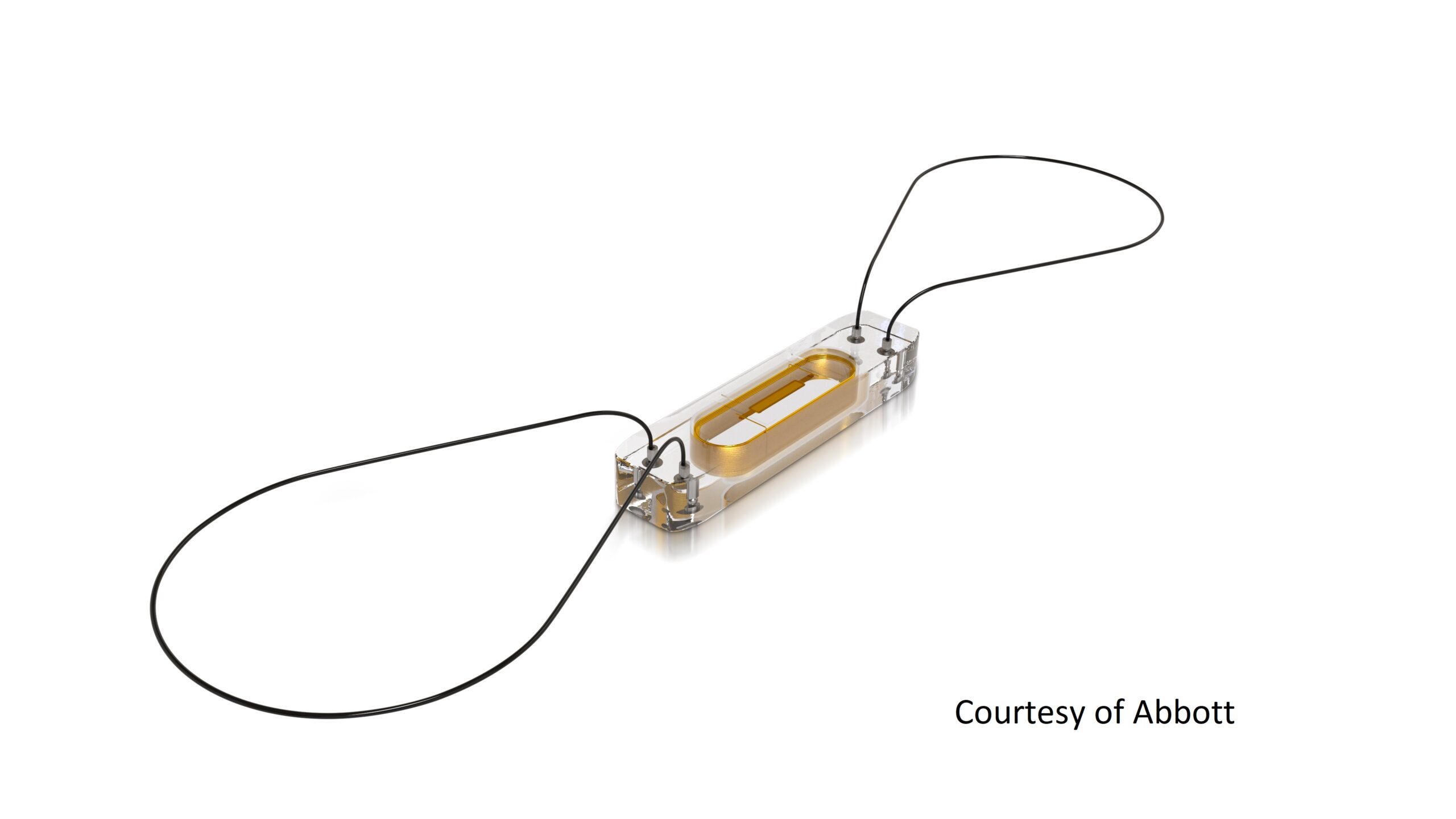
Abbott’s implantable CardioMEMS device is designed to measure pulmonary artery pressure to help prevent hospitalizations in patients with heart failure.
As Abbott seeks an expanded indication for its CardioMEMS device for patients with heart failure, it’s also hoping to win over a national coverage decision from the Centers for Medicare and Medicaid Services.
The implantable device was first approved in 2014 to prevent hospitalizations in patients with heart failure. Now, Abbott is seeking the Food and Drug Administration’s blessing to use it in a wider range of patients, including those in the earlier stages of heart failure and more severe stages of the disease.
The company filed a premarket approval supplement with FDA, and is also on CMS’ national coverage determination waitlist.
Abbott’s CardioMEMS device measures pressure on the patient’s pulmonary artery, and if they’re out of range, their doctor can adjust their medication. Although this method is more invasive, it’s a more accurate way of detecting progression than weighing patients, said Dr. Philip Adamson, chief medical officer of Abbott’s heart failure division.
The intent is to keep people out of the hospital by preventing decompensation, when fluid builds up in the lungs. Often patients don’t notice symptoms until they are far in the decompensation process, he said.
“It’s a horrible sensation. … Just to prevent this is a fantastic opportunity,” he said.
Trial results
Abbott recently tested the device in a wider range of patients, including class II to class IV heart failure patients. While CardioMEMS is currently cleared for patients with class III heart failure who have previously been hospitalized, the trial also included patients who hadn’t been hospitalized with the condition yet, but had blood test results indicative of heart failure. Like many trials last year, the study was cut short because of the Covid-19 pandemic.
The one-year study included 1,000 patients who were implanted with the device, with half of them receiving heart failure management based on pulmonary artery pressure, and the other half receiving the usual standard of care. It fell short of proving that the patients whose care involved the device saw fewer heart failure events or lower mortality rates, according to results published in the Lancet.
However, Covid-19 might have affected these results. An analysis of data prior to the pandemic found a 19% reduction in heart failure hospitalizations, ER visits and death, and a 34% reduction specifically for earlier-stage heart failure patients.
Analysts expect this should be enough evidence to support an expansion of the device for earlier stage (class II) heart failure patients. For patients with more severe (class IV) heart failure, however, patient enrollment was too small to draw any conclusion of clinical benefit, wrote Robbie Marcus, a senior analyst at JPMorgan, in an August 27 research note.
“While the trial failed to show a mortality benefit, this was unsurprising given the smaller pre- Covid-19 population and brief time-frame of only one year,” he wrote. “While there was some criticism directed at the use of the pre-Covid-19 analysis, the fact that the sensitivity analysis (p=0.11) met the pre-specified benchmark of 0.15 agreed upon with the FDA leads us to believe that this shouldn’t represent a barrier to approval.”
Coverage plans
Abbott hopes to get CMS coverage of the device for both the original and expanded indications.
“What we don’t want to do is work with them for the incomplete indication and have to go back and amend it,” Adamson said. “We want to get clarity on what the indication will be and work with them on a national coverage decision based on that indication.”
If the FDA takes a full six months to make its decision, he expects to hear back from the agency in January. Then, Abbott could begin the nine-month process with CMS.
Abbott had faced challenges with reimbursement a few years ago, with some insurers raising concerns about its pivotal CHAMPION trial. In 2016, Medicare administrative contractor Novitas declined to cover the device, saying more evidence was needed. Novitas dropped the non-coverage policy last year.
Abbott CEO Robert Ford acknowledged those challenges in a July 22 earnings call, adding that he hoped the new results would address concerns about the previous trial.
“We believe that monitoring pulmonary arterial pressure is a great indicator for prevention of acute heart failure and decided to make the investment in a larger trial to either address the perceived shortcomings of CHAMPION and/or augment the dataset,” he said.










