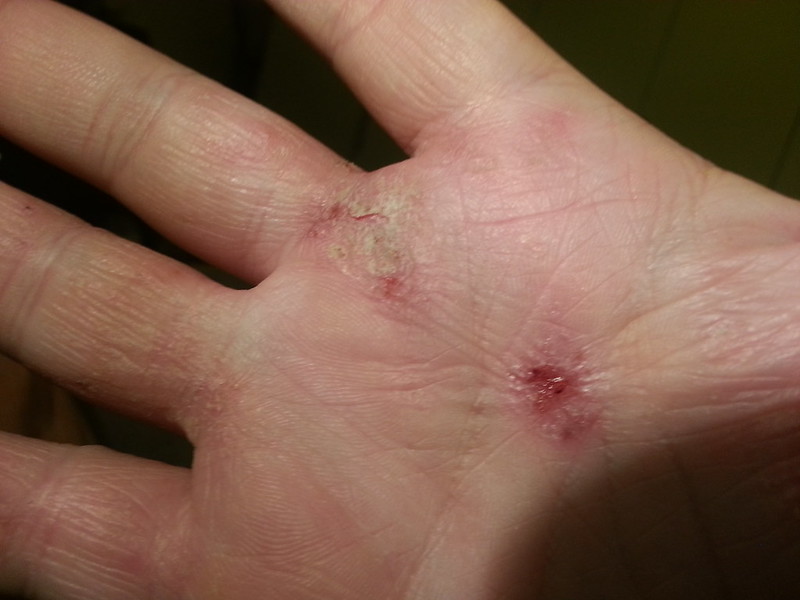
Incyte’s atopic dermatitis drug is now approved, a decision that comes three months later than expected as the topical cream fell under the renewed scrutiny placed on other drugs in the same class. Those drugs carry the FDA’s strictest warning on their labels, alerting physicians about serious safety risks, some of them potentially fatal.

The Funding Model for Cancer Innovation is Broken — We Can Fix It
Closing cancer health equity gaps require medical breakthroughs made possible by new funding approaches.
Incyte executives point out that the warning is for the class of Janus kinase (JAK) inhibitors broadly and not the company’s drug, ruxolitinib, specifically. Also, the warning follows the FDA’s review of oral JAK drugs that circulate systemically. As a topical cream applied directly to the red and itchy skin caused by atopic dermatitis, the Wilmington, Delaware-based company’s drug is designed to provide targeted and fast treatment, avoiding the systemic exposure that can spark side effects elsewhere in the body.
Speaking on a conference call Wednesday, Jim Lee, Incyte’s group vice president and head of inflammation and autoimmunity, said that clinical trial results showed that the drug, which will be marketed under the name “Opzelura,” was safe, with none of the cancer or cardiovascular risks associated with oral JAK drugs.
“We believe the safety data, as well as the limited systemic exposure observed with Opzelura, did not warrant a box warning,” he said. “Despite the class labeling, we believe Opzelura, with its robust efficacy and safety profile, will deliver a significant benefit for patient with uncontrolled atopic dermatitis.”
Atopic dermatitis, also called eczema, is a chronic autoinflammatory disorder that leads to rashes, dry skin that is vulnerable to infection, and severe itching. The condition affects an estimated 16.5 million adults and 9.6 million children in the U.S., according to the National Eczema Association. Treatment includes oral and topical steroids, but these drugs don’t work for all patients. Dupixent, marketed by Sanofi and Regeneron Pharmaceuticals, is the first biologic drug approved for atopic dermatitis. That blockbuster-selling antibody drug, given as an injection every two to four weeks, blocks two signaling pathways that play a role in inflammation.

Tips for Greater Healthcare Practice Success in 2025
Join us on February 26 at 3 pm ET, and we’ll dive into the key areas where practices are losing time and money and provide solutions to overcome them.
JAK enzymes modulate the cell-signaling proteins involved in inflammatory responses and blocking them offers another way to address autoimmune disorders. Ruxolitinib was first approved in 2011 as a treatment for myelofibrosis, a bone marrow disease. The tablet, marketed as “Jakafi,” also has approval to treat the blood cancer polycythemia vera as well as acute graft versus host disease.
Other JAK inhibitors have reached the market to address a range of autoimmune conditions, but those drugs are pills. Incyte sees the topical formulation of Opzelura as an important differentiator and the company made a huge financial investment to get the drug to the market quickly. Last September, Incyte paid $120 million to purchase a priority review voucher with plans to use it to speed up regulatory review of ruxolitinib topical cream for atopic dermatitis. These vouchers shave four months off standard review; when the FDA accepted the company’s drug application earlier this year, it set a June 21 target date for a decision. That date came and went, as the FDA postponed decisions on other JAK inhibitors until completing its safety review.
Three weeks ago, the FDA finished that inquiry, which focused on the risks of Pfizer’s Xeljanz, an oral JAK inhibitor approved for treating rheumatoid arthritis. The FDA review found a higher risk of cardiovascular problems and cancer in patients treated with the drug, leading the agency to require that additional warnings be placed on Xeljanz’s label. That requirement extends to JAK inhibitors from AbbVie and Eli Lilly.
FDA approval of Opzelura was based on results of two Phase 3 studies, each enrolling more than 600 adolescents and adults with mild-to-moderate atopic dermatitis. Patients were randomly assigned to groups given Opzelura cream or a cream containing no drug over the course of eight weeks. The main goal was to see how many achieved clear or almost clear skin as measured according to a scale used to assess skin conditions. Results showed that 53.8% of patients treated with Opzelura achieved that mark in the first Phase 3 study compared to 15.1% of those given cream with no drug. In the second Phase 3 clinical trial, 51.3% of treated patients achieved the main goal compared to 7.6% given cream with no drug.
The most common side effects reported in the clinical trials included the common cold, diarrhea, bronchitis, ear infection, a higher count of white blood cells called eosinophils. Lee said one skin cancer case was reported during the eight-week portion of the study. That cancer was in an untreated part of the body and was deemed to be unrelated to Opzelura. The cardiovascular event was in a patient who had other underlying factors. That cardiovascular problem was found to be unrelated to the treatment with Opzelura. Additional skin cancers and cardiovascular problems reported in the 44-week extension study were deemed unrelated to the study drug.
Lawrence Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, said on the conference call that most atopic dermatitis patients will be treated with topical medications, with systemic therapies being reserved for more severe cases. Eichenfield, who is also chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego, and has served as an investigator in Opzelura’s clinical trials, said that the Incyte drug will be an important one for atopic dermatitis given the percentage of patients that achieved clear or almost clear skin in clinical testing. He added that dermatologists will see the boxed warning as reflective of oral JAK inhibitors.
“What’s in the boxed warning is different than what’s seen in the Opzelura data,” Eichenfield said. “I think dermatologists are good at parsing that out.”
Approval of Opzelura covers patients 12 and older who are not immunocompromised and whose eczema is not adequately controlled with topical drugs. Todd Edwards, Incyte’s group vice president and business unit head of inflammation and autoimmunity, said the company expects insurance companies will require patients to first try other topical treatments before permitting coverage of Opzelura.
Incyte has set a $1,950 wholesale price, before any rebates or discounts, for each tube of Opzelura. The company estimates that patients will go through three to four tubes per year. For comparison, Dupixent’s wholesale price is $3,203.39 per carton, each carton containing two doses. Ruxolitinib is already a blockbuster seller for Incyte, generating more than $1.9 billion in revenue last year. The company projects that Opzelura’s annual U.S. sales will reach $1.5 billion. Incyte expects to launch the drug in the first week in October.
Photo by Flickr user Oregon State University via a Creative Commons license








