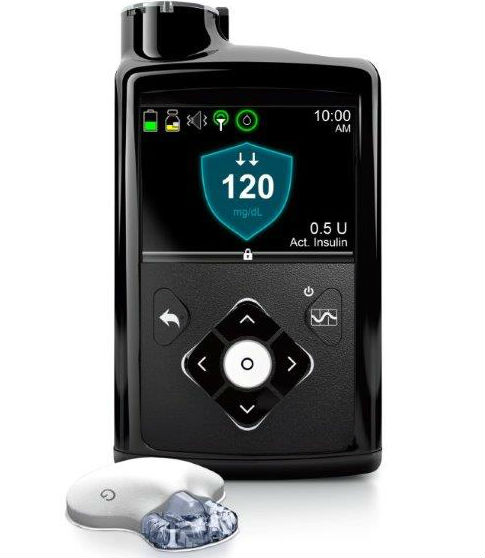
Medtronic is recalling its MiniMed 670G system after reports of broken retainer rings for insulin cartridges leading patients to receive the incorrect dose. Photo credit: Medtronic
Medtronic is expanding a recall of its MiniMed insulin pumps after a broken retainer ring led patients to receive the incorrect dose of insulin.
The recall, first announced in November 2019, was expanded yesterday (Opens in a new window)to include all Minimed 630G and 670G insulin pumps for Medtronic to replace their retainer rings. It includes more than 463,000 of the devices in the U.S.
The clear retainer ring, intended to lock an insulin cartridge into the devices, would sometimes break, resulting in patients receiving either too much or too little insulin. In one complaint filed with the FDA’s MAUDE database (Opens in a new window), a patient said they had the pump replaced for a fourth time due to a crack on the back of the pump, leading to inaccurate readings and severe low blood sugar.
In a February notice (Opens in a new window), the FDA said it is aware of 2,175 reported injuries and one death. It recently updated the recall notice (Opens in a new window) to say that serious injuries and deaths have been reported with the use of the recalled insulin pumps, but not all of those adverse events may have been directly related to the damaged retainer rings.
Some patients have sued the company over the defect. Last year, a patient with Type 1 diabetes filed a lawsuit (Opens in a new window)after a defect with her insulin pump prevented the reservoir from properly sitting in place. This resulted in it delivering its entire reservoir of insulin, causing her to pass out from hypoglycemia. Earlier this year, she reached a settlement with Medtronic.
In a statement posted to its website, (Opens in a new window) Medtronic said it is updating the recall to proactively replace the affected insulin pumps with ones that have black retainer rings designed to better withstand damage from accidental bumps or drops.
Separately, the FDA also expanded a recall (Opens in a new window) yesterday of remote controllers used with Medtronic’s MiniMed 508 insulin pumps or the MiniMed Paradigm for potential cybersecurity risks.
Medtronic is currently in the process of seeking approval for its newest insulin pump, the 780G, but like many companies, it has faced delays (Opens in a new window) as the FDA has been busy with Covid-19.










