Not long after Paige cinched FDA clearance for its algorithm to flag potentially cancerous lesions, another digital pathology startup is touting the results of a study claiming its deep learning technology can detect melanoma with a high degree of accuracy.
Philadelphia-based Proscia shared the results of a prospective study testing its DermAI software on skin biopsies from two different laboratories. It found that its AI detected melanoma in situ and invasive melanoma with a sensitivity of 93% and a specificity of 91%.

Reducing Clinical and Staff Burnout with AI Automation
As technology advances, AI-powered tools will increasingly reduce the administrative burdens on healthcare providers.
Proscia has not yet published the results of the prospective study, but it hopes to move toward using its software in a clinical setting. Currently, DermAI is only for research use.
In an emailed statement, Proscia vice president of research and development Julianna Ianni wrote that the company was eager to introduce DermAI in routine practice.
“We are taking the steps necessary to do so, including conducting ongoing validation studies,” she wrote.
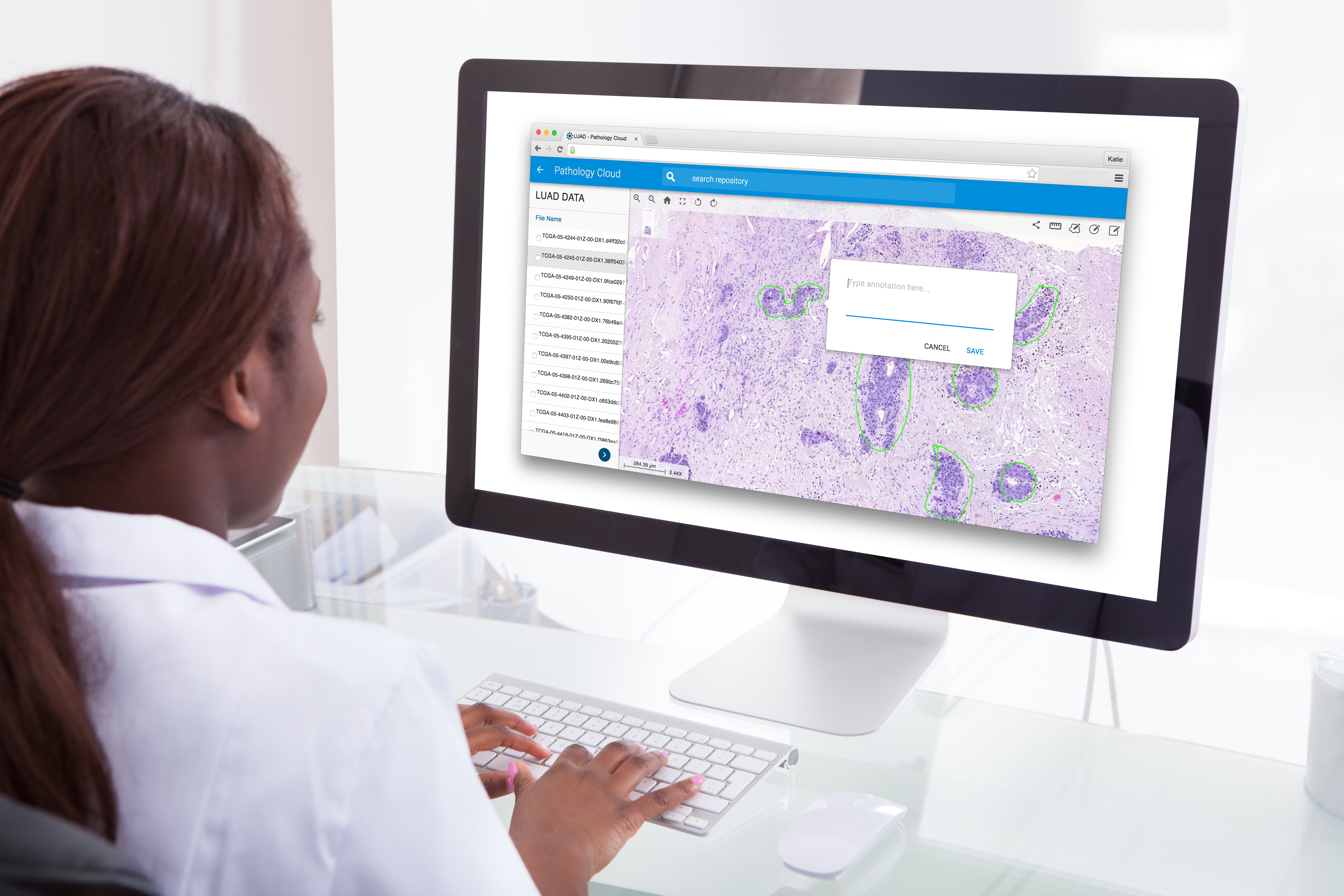
For the prospective study, the AI was run on 1,422 skin biopsies that were scanned to create whole slide images. The software would run, and then a pathologist would review a case, with the ability to sort or prioritize cases based on the AI’s classification.
For example, if the AI identified a potential case of melanoma, the pathologist could urgently review it ahead of other cases, wrote Dr. Kiran Motaparthi, Director of Dermatopathology at the University of Florida, who helped lead the study.
The biopsies from both labs were uncurated and anonymized, to “ensure that the data we used was representative of what these laboratories would encounter in day-to-day operations,” he wrote.
Motaparthi noted that there was variation in patient demographics, diagnoses, and the way any diagnosis would appear, as well as how biopsies are prepared and stained. However, Proscia did not share details on patient demographics for the study.
It builds on a previous retrospective study conducted by Proscia, where it trained the system on more than 7,600 images from one lab, and tested it on a little over 5,000 from two other labs.
Proscia has additional tests planned to see if its AI can deliver results faster for patients with melanoma, and if it can make diagnosis more consistent. It might also benefit from Paige’s de novo clearance, which opened a new path for other digital pathology companies looking to get the green light for their solutions from the FDA.
Last year, Proscia raised $23 million, with the intent of getting regulatory clearance for more of its products. Its software to scan, store and analyze tissue biopsies has been CE marked in Europe. It also made it available in the U.S., under a temporary guidance by the FDA to allow pathologists to use these devices to review scanned slides remotely, minimizing their exposure to Covid-19.
Photo credit: Proscia