Thus far, the promise of precision medicine has been directed largely toward the field of oncology in terms of recommending personalized treatments based on molecular diagnostics tests. But increasingly, the knowledge of if specific individuals will or won’t benefit from certain treatments is informing providers in a broader set of diseases.
On Monday, Scipher Medicine, a precision immunology company based in Waltham, Massachusetts, announced clinical results of a study that examined treatment and outcomes for 212 rheumatoid arthritis (RA) patients whose healthcare providers used its PrismRA blood test. Specifically, the blood test was used to predict non-response to the world’s largest drug class — tumor necrosis factor inhibitor (TNFi).

NEMT Partner Guide: Why Payers and Providers Should Choose MediDrive’s TMS
Alan Murray on improving access for medical transportation.
The Study to Accelerate Information of Molecular Signatures (AIMS) found RA patients whose PrismRA blood test indicated that they would not respond to TNFi therapy, but received it anyway, did not respond 90% of the time. Meanwhile, patients whose treatment was guided by PrismRA had three times better clinical responses compared to patients that didn’t. The results of Scipher’s study were published in Expert Review of Molecular Diagnostics.
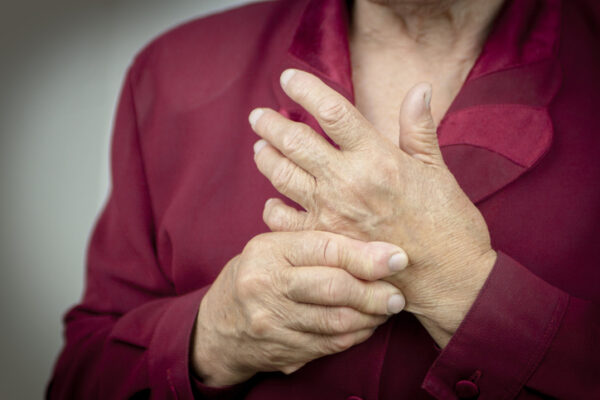
Rheumatoid arthritis is a form of arthritis that causes pain, swelling, stiffness and loss of function in joints. While it can affect any joint, it is more commonly observed in the wrist and fingers. It is incurable.
In an email, Sam Asgarian, chief medical officer of Scipher Medicine, explained that the gold standard for measuring clinical outcomes in rheumatoid arthritis is the ACR50 measurement. Created by the American College of Rheumatology, it is a series of composite scores measuring improvement in symptoms by at least 50% in the number of swollen joints and number of tender joints. The same 50% or more improvement also has to be demonstrated in three of five measures including surveys on physician and patient assessment and other clinical and lab results.
Asgarian said that Scipher’s CMS-approved lab test is the only commercially available blood test that predicts patient response to TNFi therapies.

The Power of One: Redefining Healthcare with an AI-Driven Unified Platform
In a landscape where complexity has long been the norm, the power of one lies not just in unification, but in intelligence and automation.
The AIMS study evaluated data from dozens of private and academic rheumatology practices and U.S. institutions including Stanford University, Texas Health Presbyterian Hospital in Dallas, University of Alabama at Birmingham, and the University of South Florida.
“This clinical study makes clear that a blood test capable of predicting patient responses to a commonly prescribed class of rheumatoid arthritis therapies could fundamentally shift the treatment paradigm,” said study co-author Dr. Vibeke Strand, adjunct clinical professor, Division of Immunology and Rheumatology, Stanford University School of Medicine, in a news release. “Broad adoption of this important advancement could result in significant improvements in treatment outcomes.”
Should Schipher’s PrismRA become part of routing care, it is likely to significantly improve more than just patient outcomes. That’s because there is a financial burden of failed treatments especially if the company’s claim that two-thirds of patients taking TNFi therapies do not respond clinically is accurate. In his response to questions, Asgarian explained that a retrospective study of RA patients found that “patients who respond to their targeted therapy incur on average $6,091 less per year in total medical costs (not including the cost of targeted therapy) than non-responders.”
That’s because they have fewer inpatient and ED admissions, fewer outpatient visits, lower spending on antidepressants and pain medication, among other factors.
“PrismRA can therefore help payers and patients save on both pharmacy and medical costs,” he said. “On the pharmacy side, rather than placing a patient requiring biologic prescription therapy on a TNF inhibitor by default, a PrismRA test result may lead the provider to prescribe an alternate, less costly therapy at the onset.”
Even though there are five approved classes of targeted therapy for RA, an estimated 90% of patients are prescribed TNFi therapies, according to Scipher Medicine. Some common TNFi therapies for RA include AbbVie’s Humira; Amgen/Immunex’s Enbrel; and Janssen’s Remicade.
While Scipher’s trial results are promising, the success of PrsimRA will be contingent upon winning insurance reimbursement. Currently, the company is awaiting a Local Coverage Determination from Medicare, which would enable it to get reimbursement in federal health plans. It is also working with more than 20 companies — private health insurers and pharmacy benefit managers — to win broad access and coverage.
RA is not the only disease the company is targeting. Asgarian said that the company hopes to introduce at least one test annually over the next five years in the field of autoimmune diseases. The company has plenty of capital to support these endeavors. Asgarian said Scipher has raised $117 million in three financing rounds since its inception in 2015. Investors in the company include UnitedHealth Group, Optum, Alumni Ventures, aMoon, Northpond Ventures, ECHO and Khosla Ventures.
Photo: Sue777, Getty Images






