
Name of Company: UltraSight
Davidi Vortman, CEO of the Israeli company, described details of the FDA clearance received on July 27.
Name of the Product:
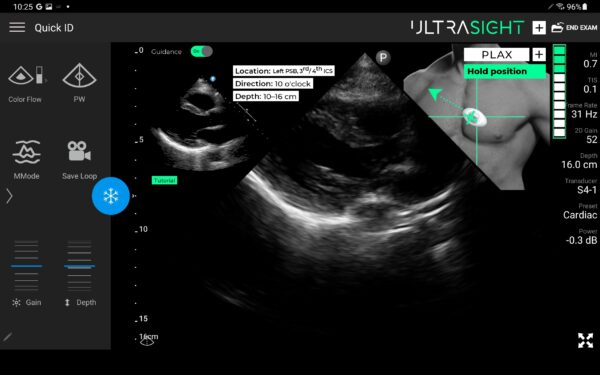
UltraSight AI Guidance
Describe the problem that the product addresses.
There is a tremendous need for timely and consistent cardiac ultrasound, especially given the prevalence of heart disease in the United States. The UltraSight AI Guidance can assist medical professionals without sonography experience in acquiring cardiac ultrasound images and allow for more widespread detection of heart diseases. It can provide patients easier access to cardiac monitoring and help alleviate bottlenecks in the healthcare system. According to the CDC, more than 8 million Americans are admitted to the ER every year with symptoms of heart attack or heart failure, while approximately 30 million heart disease patients require cardiac monitoring.
Who is the end-user?:
The UltraSight real-time AI guidance software can assist medical professionals without sonography experience in acquiring cardiac ultrasound images at the point of care in multiple settings.

The Funding Model for Cancer Innovation is Broken — We Can Fix It
Closing cancer health equity gaps require medical breakthroughs made possible by new funding approaches.
Does the product need reimbursement and is it covered?
Yes, there is an existing code for point of care ultrasound (93308)
What are the competitive products on the market?:
Caption Health (recently acquired by GE Healthcare) and EchoNous (UltraSight recently partnered with EchoNous)
Photo: jayk7, Getty Images










