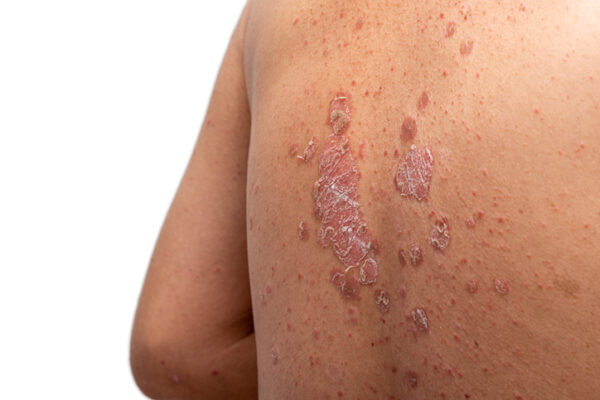
A UCB drug with a novel approach to plaque psoriasis won a long-awaited FDA approval, positioning it to bring patients a new choice in a crowded market of products for the prevalent skin condition. The Belgian drugmaker on Wednesday also won a separate regulatory nod for a different drug, giving it another approved treatment for a rare muscle disorder.
The regulatory decision for the plaque psoriasis drug, bimekizumab, covers the treatment of adults who have a moderate-to-severe form of the autoinflammatory disease. UCB said Wednesday that the subcutaneously injected drug will be marketed as Bimzelx.

With the Rise of AI, What IP Disputes in Healthcare Are Likely to Emerge?
Munck Wilson Mandala Partner Greg Howison shared his perspective on some of the legal ramifications around AI, IP, connected devices and the data they generate, in response to emailed questions.
In plaque psoriasis, an excessive immune response leads to the formation of red, scaly patches on the skin that can be itchy and painful. The disease affects an estimated 7.5 million adults in the U.S. The first line of treatment is typically topical steroids. Biologic drugs are options for those who develop problems from steroids or have more severe cases of the disease.
Bimzelx is an antibody designed to block IL-17A and IL-17F, signaling proteins that drive inflammation in plaque psoriasis. While other biologic drugs block proteins associated with plaque psoriasis, FDA approval of the new UCB drug makes it the first one to pass regulatory muster for targeting IL-17A and IL-17F.
The FDA decision for Bimzelx is based on Phase 3 data showing that the UCB drug achieved better skin clearance compared to both a placebo or Stelara, a blockbuster Johnson & Johnson plaque psoriasis antibody designed to block two other proteins, IL-12 and IL-23. Bimzelx’s FDA nod comes two years later than expected. In 2021, the FDA deferred a decision, citing the inability to inspect the manufacturing facilities for the drug due to Covid-19 travel restrictions—a reason commonly cited during the pandemic. The agency turned down UCB’s application last year, again citing the inability to conduct the necessary manufacturing inspection.
In the time since, other plaque psoriasis medications have entered the market. Sotyktu, a small molecule from Bristol Myers Squibb designed to block a protein called TYK2, was approved last year. There have also been innovations in steroid-free topical alternatives. Dermavant Sciences’ Vtama and Zoryve from Arcutis Biotherapeutics are two such drugs. UCB’s new plaque psoriasis med will soon join them on the market. The company said its new drug will become available in the U.S. in about one month.
FDA Nod Gives UCB Chance to Challenge Two AstraZeneca Meds
The other UCB drug approved Wednesday treats generalized myasthenia gravis, giving the company a second medication to pass FDA muster in this indication this year. Generalized myasthenia gravis is an immune disorder that develops from the formation of autoantibodies that interfere with communication between nerves and muscles. The disease can be treated with older drugs, such as corticosteroids and immunosuppressants.
A newer approach to the disease involves tackling the autoantibodies with antibody drugs. In 2021, Netherlands-based Argenx won the first FDA approval of such a drug with Vyvgart. This antibody fragment has the effect of leading to the autoantibodies being broken down by a built-in cellular protein disposal system. UCB won FDA approval in June for Rystiggo, an antibody that works similarly to Argenx’s drugs.
The Wednesday approval of zilucoplan, brand name Zilbrysq, gives UCB another approach to myasthenia gravis. Zilbrysq is a peptide designed to block C5, a protein in the complement system, a part of the immune system. The drug is intended to stop complement-mediated damage to the neuromuscular junction, which is a synaptic connection between the end of a motor nerve and a muscle. Two C5 inhibitors are already available for myasthenia gravis. Soliris and Ultomiris, both from AstraZeneca subsidiary Alexion, are monoclonal antibodies with approvals that include this indication.
Zilbrysq joined the UCB pipeline via the $2.1 billion acquisition of Ra Pharmaceuticals four years ago. As a peptide, Zilbrysq has some advantages compared to the two AstraZeneca antibodies. Some myasthenia gravis patients undergo therapeutic plasma exchange, an outpatient procedure in which a machine withdraws blood and removes the problem antibodies, then returns the plasma and other blood components to the body. UCB contends that unlike antibody drugs, Zilbrysq’s peptide composition allows it to be used alongside plasma exchange without the need for supplemental dosing. The UCB drug also offers an easier dosing option for patients. The AstraZeneca drugs are intravenous infusions that must be administered in a healthcare setting. UCB developed Zilbrysq as a once-daily subcutaneous injection that can be self-administered via a single-dose, prefilled syringe.
“Until now, people living with [generalized myasthenia gravis] have only had access to C5 therapy intravenously, which can be inconvenient and time consuming,” Iris Loew-Friedrich, UCB executive vice president and chief medical officer, said in a prepared statement. “Now, with the option of Zilbrysq, a self-administered once-daily, subcutaneous targeted complement C5 inhibitor, we hope a broad population of mild-to-severe adult patients with AChR-antibody-positive [generalized myasthenia gravis] will be able to have greater independence.”
FDA approval of Zilbrysq is supported by a placebo-controlled Phase 3 study with results that showed statistically significant benefit after 12 weeks of treatment. The most common adverse reactions were injection site reactions, upper respiratory tract infections, and diarrhea. The drug’s label carries a black box warning that life-threatening and fatal meningococcal infections have developed in patients treated with complement inhibitors. To mitigate those risks, patients must be up to date on their meningococcal vaccinations at least two weeks before dosing with this UCB drug. The drug labels of other C5 inhibitors carry similar warnings. Zilbrysq is available only under a program that alerts physicians and patients about these risks.
Photo: Getty Images














