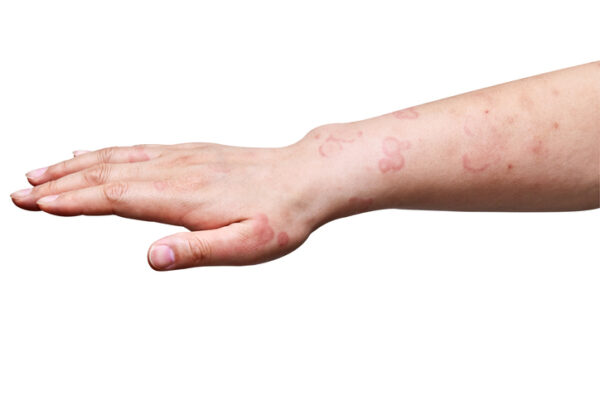
Only one biologic drug is approved for treating the hives and itchy rash caused by chronic spontaneous urticaria, an inflammatory skin disorder. Celldex Therapeutics’ lead program is shaping up as a viable and potentially superior alternative.
The biotech reported preliminary Phase 2 data on Monday, showing its biologic, barzolvolimab, met the goals of its mid-stage study. In the placebo-controlled clinical trial, all three doses of the study drug showed statistically significant and clinically meaningful decreases according to an assessment that scores symptoms of urticaria, such as hives and itching. No severe adverse effects were reported. Based on these results, Hampton, New Jersey-based Celldex said it plans to advance barzolvolimab to Phase 3 testing in 2024.

With the Rise of AI, What IP Disputes in Healthcare Are Likely to Emerge?
Munck Wilson Mandala Partner Greg Howison shared his perspective on some of the legal ramifications around AI, IP, connected devices and the data they generate, in response to emailed questions.
Urticaria is another term for hives. In chronic spontaneous urticaria, these hives have no known trigger and they last for six weeks or longer. Antihistamines comprise the first line of therapies for the condition. When those drugs don’t work, patients may take Xolair from Roche subsidiary Genentech. This antibody drug is designed to block immunoglobulin E, preventing these immune system-produced antibodies from binding to mast cells and basophils, two types of immune cells.
Barzolvolimab, or barzo for short, also addresses mast cells, but it works in a different way. The Celldex drug is designed to bind to KIT, a receptor that regulates a variety of cells, including mast cells. Blocking KIT is intended to interfere with the role mast cells play in driving allergic and inflammatory conditions.
The Celldex drug is administered as a subcutaneous injection. The Phase 2 study enrolled 208 patients whose chronic spontaneous urticaria persisted despite treatment with antihistamines. The study tested a low dose and a middle dose administered every four weeks. A high dose given every eight weeks was also evaluated.
Barzo’s middle dose had the best outcome, with 51.1% of patients achieving complete control of their urticaria symptoms. That result compares favorably against other drug candidates for chronic spontaneous urticaria. With the usual cross-trial comparison caveats, Leerink Partners analyst Thomas Smith said in a Monday research note that barzo’s complete response result is better than any competitor’s Phase 2 or Phase 3 data to date, including Xolair as well as two from Novartis: the BTK-blocker remibrutinib, which is in Phase 3 development, and ligelizumab, an immunoglobulin E-blocking antibody that was discontinued after failing to beat Xolair. Smith added that the Celldex drug’s results are more impressive given that about 20% of patients in that drug’s Phase 2 study previously received Xolair and did not achieve an adequate response to that therapy.
“Overall, we see this as a strong [chronic spontaneous urticaria] dataset, with signals of best-in-class efficacy in a difficult-to-treat patient population that includes 20% Xolair-experienced patients,” Smith said.
In a statement, Celldex President and CEO Anthony Marucci said the results so far reinforce barzo’s approach targeting a key underlying pathway of chronic spontaneous urticaria. He added that full data from the clinical trial will be presented at an upcoming medical meeting.
Barzo’s Phase 2 results in urticaria came on the heels of the release of encouraging preliminary Phase 1b data from a test of the drug in prurigo nodularis, a different skin disorder that leads to the formation of hard, itchy bumps on the skin. The main goal of this test was to assess the drug’s safety. Results showed that adverse events were mild to moderate and were unrelated to the study drug. Secondary goals included assessing itch scores. Celldex reported that a single intravenous dose of barzo led to “rapid and durable reductions in itch and healing of skin lesions.” In this indication, Celldex is planning to advance to a Phase 2 study in 2024 that will test multiple subcutaneous doses.
Barzo is also in clinical development in a third indication. Over the summer, Celldex enrolled its first patient in a Phase 2 test of the drug in eosinophilic esophagitis, a chronic inflammatory condition affecting the esophagus.
Celldex isn’t the only biotech aiming to treat inflammatory disorders by blocking KIT. San Francisco-based Third Harmonic Bio is developing small molecules that target the receptor. Nearly a year ago, a KIT inhibitor that Third Harmonic licensed from Novartis posted preliminary Phase 1b data showing liver problems in some patients. The biotech subsequently discontinued that program. Another KIT-blocking small molecule called THB335 is in development for mast cell-mediated conditions. Third Harmonic has said it expects to begin a Phase 1 clinical trial for this program in the first half of 2024.
Photo: Getty Images














