The 43rd Annual J.P. Morgan Healthcare Conference (JPM), which took place Jan. 13 through 16 in San Francisco, is one of the largest healthcare investment symposiums in the industry, connecting global industry leaders, emerging fast-growth companies, innovative technology creators, and members of the investment community.
Below are three key topics that emerged from conversations on the ground floor.
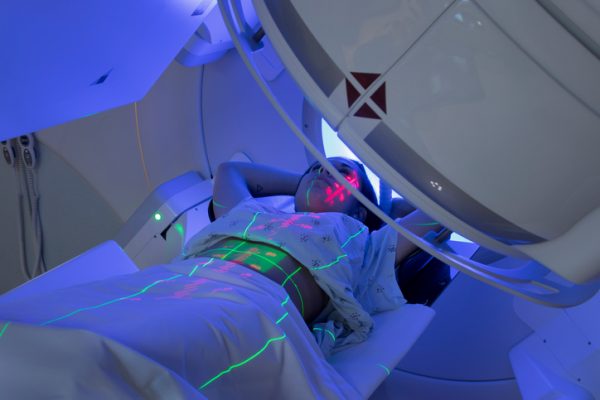
Radiotherapy on the rise

As Healthcare and Biopharma Companies Embrace AI, Insurance Underwriters See Risks and Opportunities
In an interview, Munich Re Specialty Senior Vice President Jim Craig talked about the risk that accompanies innovation and the important role that insurers play.
Radiotherapy was a key focus among attendees. Radiotherapy, also known as radiation therapy, uses targeted radiation to kill cancer cells and shrink tumors. The radiotherapy market is growing rapidly due to a focus on advancements in radiotherapy treatment technologies, a growing cancer patient population, the increasing use of particle therapy for cancer treatment and increased initiatives to promote radiotherapy awareness, according to a December 2024 report from MarketsandMarkets, a market research firm. The report concluded that the global radiotherapy market is projected to grow from $7.21 billion in 2024 to $9.62 billion by 2030, a CAGR (compound annual growth rate) during the growth period of 4.9%.
Many investors and strategic partners were already aware of the clinical and commercial importance of radiotherapy in the oncology space, but this awareness has been heightened by positive study results, new FDA approvals, and growing commercial traction following the clinical and commercial successes of radiopharmaceuticals such as Lutathera and Pluvicto and the increase in mergers and acquisitions (M&A) activity. Lutathera (lutetium Lu 177 dotatate) has demonstrated promise as part of the initial therapy for some neuroendocrine tumors (NETs) and is the first radioactive drug approved to treat these rare tumors. Pluvicto (lutetium Lu 177 vipivotide tetraxetan) is a radioactive drug that has demonstrated significant benefits in patients with metastatic prostate cancer. A wave of radiopharmaceutical companies has arisen to offer competition to Lutathera and Pluvicto in those two indications. With regard to M&A activity, TaylorWessing, a global law firm specializing in technology and life sciences, has identified therapeutic radiopharmaceuticals for oncology indications as a treatment modality that has recently built up “real traction in the market.” Examples include RayzeBio, acquired in 2024 by Bristol Myers Squibb, and POINT Biopharma, acquired in late 2023 by Eli Lilly & Co. The uptick in M&A activity is also fueling investments, as funds from successful exits are being reinvested into newer companies.
China’s biotech surge
Another theme of the conference was the appetite of U.S. pharmaceutical companies for deals with China. More than a third of therapeutic molecules purchased by U.S. pharma companies in 2024 were sourced from China, compared to zero four years ago. That striking statistic is from Stifel’s Biopharmaceutical Outlook for 2025 report, released earlier this month. These deals were larger than average and three or four or more involved partnerships or whole new companies forming around a China-developed asset. Moreover, a Chinese company is involved in at least a fifth of development programs across the entire industry’s total clinical pipeline, according to a 2025 preview report from Evaluate Pharma. Venture companies are following suit, launching U.S. startups around Chinese-sourced compounds. Historically, Chinese deals have focused on oncology, but pharmaceutical company prospecting has broadened to include obesity, immunology and cardiometabolic diseases.
Stimulated by government investment in biotech R&D, efforts to make its business climate more biotech-hospitable, and an influx of U.S. and European-trained scientists, China’s biotech boom is threatening the global dominance of the U.S. pharmaceutical industry and raising concerns about its reliance on Chinese R&D for new drugs. An example cited in BioPharma Dive is ivonescimab, a drug developed by China-based Akeso and licensed by U.S.-based Summit Therapeutics. Recent results from a lung cancer study conducted in China showed that ivonescimab outperformed Keytruda. Manufactured by Merck, Keytruda is its dominant immunotherapy and is currently the pharmaceutical industry’s most lucrative product. Boris Zaïtra, head of business development at Roche, which sells a rival to Keytruda, was quoted as saying that the finding “put a huge focus on what’s happening in China.”
Whether the trend will hold is a matter of speculation, but in the meanwhile, it serves as a warning to the U.S. pharmaceutical industry that it is facing some stiff competition when it comes to innovation.
AI and drug discovery
Though AI’s role in drug discovery has yet to play out, the enormous potential upside in terms of savings of time, resources, and money was of great interest to conference participants. A 2024 study in JAMA Network Open, for instance, found that the mean cost of bringing a drug to market from 2000 to 2018 was $172.7 million (in 2018 dollars) in a process that typically takes 10 to 15 years. Even small improvements in these numbers would be a boon to drug discovery and patient health. In particular, participants shared their hopes that AI will help cut the failure rate for new drugs. Despite the significant investments mentioned above, about 90% of drugs in clinical development still fail. When the cost of failures was included in the JAMA Network Open study, the cost of bringing a drug to market increased to $518.8 million and, when both drug development failure and capital costs were included, to $879.3 million, or about $1.1 billion in today’s dollars.
The huge amounts of data amassed by pharmaceutical companies over decades are ripe for mining by AI companies, many of whom are partnering with big pharmaceutical companies in the discovery and design of new small molecules. At the conference, the tech giant NVIDIA, the world’s most valuable company, for example, announced new partnerships to transform the healthcare and life sciences industry by using AI to speed clinical trials by reducing administrative burden, advancing drug discovery and enhancing genomic research. The partnerships announced included those with IQVIA, a global provider of clinical research services, commercial insights and healthcare intelligence to the life sciences and healthcare industries; Illumina, a global leader in DNA sequencing and informatics technologies; and Arc Institute, a research organization operating at the intersection of biology and machine learning.
Photo: Getty Images, 501972854
Raphi Levy specializes in corporate finance and strategy and has served as Alpha Tau’s CFO since 2019, after spending 13 years working in the investment banking division at Goldman Sachs in New York and Tel Aviv. Most recently, he served as executive director in charge of healthcare banking in Israel. He holds a bachelor’s degree in economics from The Wharton School of the University of Pennsylvania and bachelor’s and master’s degrees in electrical engineering from the University of Pennsylvania School of Engineering and Applied Science.
This post appears through the MedCity Influencers program. Anyone can publish their perspective on business and innovation in healthcare on MedCity News through MedCity Influencers. Click here to find out how.









