In gout, the drug choices for patients remain limited. The history of this field includes many molecules that fell short in the clinic or on the market for safety reasons. James Mackay knows firsthand having led a company that developed two of those withdrawn products.
Mackay is back to try again. He is now CEO of Crystalys Therapeutics, whose main asset is an in-licensed gout drug candidate with validation from its approval in several Asian countries and additional safety data from real-world use in those regions. On Tuesday, Crystalys revealed $205 million raised for Phase 3 tests that could support plans to eventually bring this small molecule to patients in the U.S. and other markets.
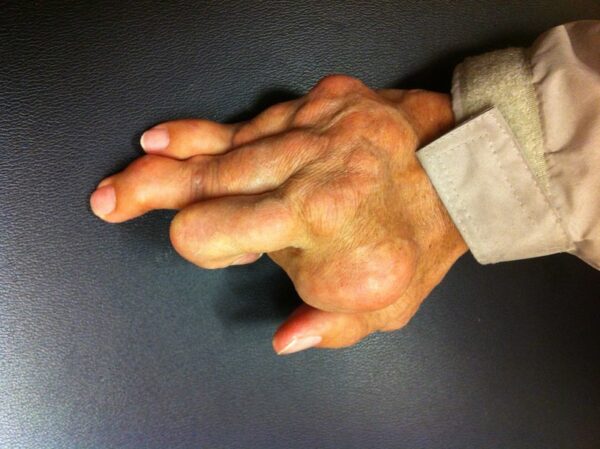
Gout, a common form of inflammatory arthritis, stems from high uric acid levels in the blood that forms crystals in joints and surrounding tissue, causing inflammation and pain. The disorder is characterized by sudden attacks of pain and swelling in the joints called gout flares. Patients with uncontrolled gout for a long period of time develop deposits of uric acid in the joints, which is called gout tophus. These tophi may not be painful, but they can impair joint movement and appear disfiguring.
The standard drug treatment for gout is allopurinol, a 60-year-old medication that inhibits an enzyme key to producing uric acid. This pill helps only about 40% of gout patients, Mackay said. San Diego-based Crystalys takes a different approach with its drug, dotinurad. The oral small molecule is designed to block URAT1, a protein responsible for the reabsorption of uric acid in the kidney. Normally, people excrete excess uric acid in their urine. Dotinurad is intended to help gout patients do that.
“If you block the transporter [protein], you basically urinate out more uric acid,” Mackay said. “What’s nice about its mechanism of action is it directly impacts the main cause of gout, which is the inability to excrete enough uric acid. Basically, it will cause you to excrete more and therefore reduce the level of uric acid in the body.”
Mackay is familiar with URAT1 inhibitors because he developed them at a previous company, Ardea Biosciences. Ardea’s lead drug candidate, lesinurad, reached Phase 3 development in gout when the biotech was acquired by AstraZeneca in 2012 for about $1 billion. In 2015, the FDA approved the drug, which reached the market under the brand name Zurampic. That drug and the combination of lesinurad and allopurinol, branded as Duzallo, were later withdrawn from the market for business reasons. Mackay said renal toxicity risks made commercializing those drugs challenging. He does not expect those problems for dotinurad, which has a safety and efficacy profile defined by its use in more than 1.2 million gout patients in Japan.
Dotinurad was discovered and developed by Japanese company Fuji Yakuhin, which steered the molecule to a regulatory approval in its home country in 2020. Eisai holds rights to the drug in several other Asian countries. In 2021, Fortress Biotech subsidiary Urica Therapeutics acquired U.S. and European rights to dotinurad; the following year, the license agreement was expanded to the Middle East and North Africa. Urica advanced dotinurad as far as Phase 1 testing in healthy volunteers and gout patients.
Mackay said Novo Ventures, which had invested in another company he had led, approached him with interest in entering the gout space. Given Mackay’s prior work in gout, they wanted his experience and expertise.
“We basically went out and did a landscape search of all the molecules under development,” he said. “We identified dotinurad as the one that we felt had the best safety and efficacy profile. And we approached Urica Therapeutics and ultimately persuaded them that we should do an asset purchase of the license from Fuji.”
In July 2024, Urica sold rights to dotinurad to Crystalys for 35% of the young biotech’s outstanding equity, according to Fortress regulatory filings. The agreement also puts Urica in line for a 3% royalty on future net sales of the drug.
Dotinurad could help fill the gap in second-line gout treatment, where treatment options are dwindling. Besides the market withdrawal of Ardea’s drugs, Takeda Pharmaceutical’s febuxostat, brand name Uloric, is exiting the market. This drug, which offers a mechanism of action similar to allopurinol, won FDA approval in 2009. A decade later, the FDA added a black box warning to Uloric’s label flagging the higher risk of cardiovascular complications, including death. Takeda has decided to discontinue the product, which currently faces generic competition. According to FDA records, Takeda will cease Uloric distribution in March 2026.
Amgen drug Krystexxa is available as a third-line gout treatment. This medicine is an engineered version of the enzyme that breaks down uric acid. But Krystexxa must be administered intravenously. Another limitation is that patients’ immune systems can produce antibodies that eventually render the biologic drug ineffective.
There are other companies developing URAT1 inhibitors that could potentially compete with Crystalys’s dotinurad. San Diego-based Arthrosi Therapeutics’ contender is AR882; China-based Atom Therapeutics is developing ABP-671. Both are in Phase 3 development. If those drugs are based on the same molecular backbone as Adrea’s withdrawn Zurampic, they will likely have the same renal toxicity issues, Mackay said. Better safety for dotinurad is part of the design of the molecule.
Research in URAT1 inhibition to treat gout dates to the 1970s, with the development of a molecule called benzbromarone. This drug won regulatory approvals in some countries, but never the U.S. Benzbromarone was hampered by reports of liver toxicity. Mackay said Fuji Yakuhin’s chemists designed dotinurad to remove the renal toxicity issues seen with previous URAT1 inhibitors. Their work also removed the hepatotoxicity observed with benzbromarone.
Dotinurad is highly specific to the URAT1 protein, leaving other renal transporters unaffected, Mackay said. Consequently, there’s a more controlled release of uric acid over 24 hours. Other URAT1 inhibitors have shown a spike in uric acid excretion, Mackay said. Pushing out too much uric acid through the kidney in a short period of time results in crystallization of uric acid that damages the kidney, he explained.
“We believe [dotinurad] doesn’t have that liability and there’s no requirement in the Japanese or the Chinese label for renal monitoring,” Mackay said. “And obviously the Japanese have given it to over a million patients since launch and no renal toxic signal has been identified.”
Crystalys’s financing, a Series A round, was co-led by Novo Holdings, SR One, and Catalys Pacific. Other participants include Perceptive Xontogeny Venture Funds, Lightstone VC, AN Venture Partners, funds managed by abrdn Inc., KB Investments, Pontifax, Longwood Fund, Alexandria Venture Investments, Wedbush Healthcare Partners, and Prebys Ventures Fund.
With the new capital, Crystalys will advance dotinurad straight to two Phase 3 studies, one assessing the drug’s effect on gout flares and the other testing tophus resolution. Given the extensive clinical trial and real-world data for the drug in Asia, the FDA is permitting Crystalys to skip Phase 2 testing, Mackay said. The only stipulation is that the FDA wants to see some level of dose ranging in the Phase 3 program, so one of the studies will evaluate a 2 mg dose and a 4 mg dose. The studies have a targeted enrollment of about 750 patients total. Mackay expects data from these trials in late 2027.
Photo by Flickr user handarmdoc via a Creative Commons license










