
Funding roundup: GRAIL raises $390M for cancer liquid biopsy test
GRAIL, a startup developing cancer liquid biopsy tests, raised a $390 million series D round. Read more about which companies raised funding this week.

GRAIL, a startup developing cancer liquid biopsy tests, raised a $390 million series D round. Read more about which companies raised funding this week.
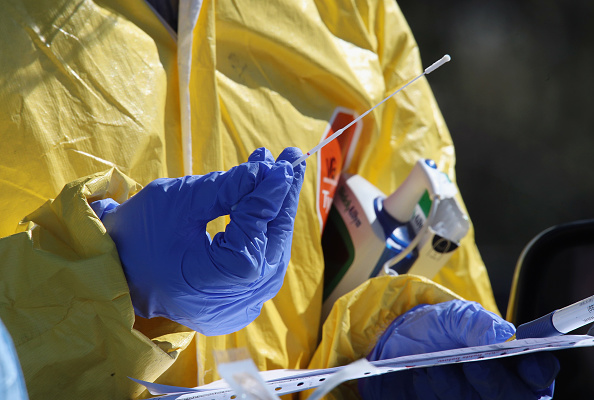
New guidance from the FDA this week would make it easier for patients to self-test for Covid-19. But the agency still hasn’t approved home testing.

Everlywell, Nurx, and a bevy of other companies had begun marketing at-home tests as the U.S. still faces a shortage. But they had to stop after the Food and Drug Administration clarified that it had not approved at-home testing.

The new capital will be directed at growing the New York-based company's at-home testing platform and boosting its manufacturing and distribution capabilities.

CEO and founder Julia Cheek started EverlyWell after her own experiences trying to diagnose her own health issues highlighted problems of cost and convenience in the traditional lab testing process.