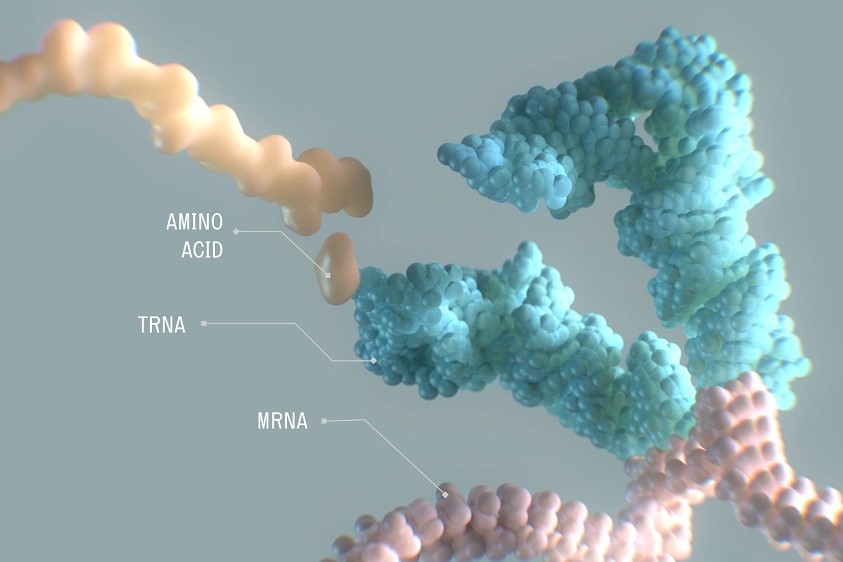
Amylyx Withdraws ALS Drug From Market; Restructuring Will Slash 70% of Staff
The voluntary removal of ALS drug Relyvrio from the market comes with a corporate restructuring that turns Amylyx Pharmaceuticals’ focus to other neurodegenerative diseases. But the company also has another ALS drug candidate set to begin clinical testing this year.