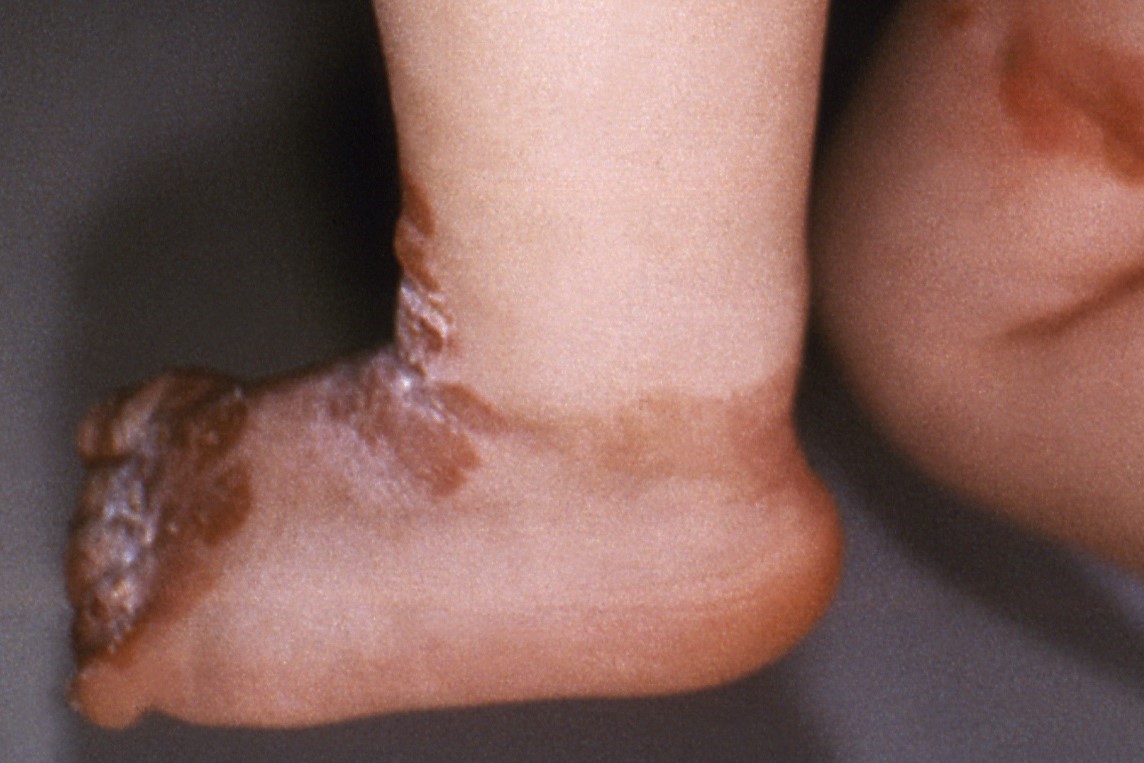
FDA Approves Abeona Gene Therapy for Healing Wounds From Rare, Inherited Skin Disease
Abeona Therapeutics makes Zevaskyn by engineering a patient’s biopsied skin cells to express the collagen that they lack, then growing those cells into sheets that can be surgically applied to a wound. Abeona's cell-based gene therapy is just the third FDA-approved treatment for epidermolysis bullosa, a rare disease that leads to skin described as thin and fragile like a butterfly’s wings.