
GW Pharmaceuticals’ cannabis-derived epilepsy drug wins European approval
The European Commission approved GW Pharma's Epidyolex as a treatment for seizures in two rare and severe forms of epilepsy. The drug won FDA approval last year.

The European Commission approved GW Pharma's Epidyolex as a treatment for seizures in two rare and severe forms of epilepsy. The drug won FDA approval last year.

The drug showed positive results in a 210-patient study in patients experiencing treatment-resistant seizures from tuberous sclerosis complex, a rare form of epilepsy.

The company's cell phone-sized device delivers low-level electric stimulation to the trigeminal nerve through a patch placed on a patient's forehead.

Cerevel will take on several clinical and preclinical candidates from Pfizer, which divested from neuroscience this year and will take a 25 percent stake in the new company.
System1 Biosciences' platform combines stem cell lines from patients with brain diseases with a three-dimensional in vitro system and data analytics.
The FDA approved the cannabis-based drug for treating seizures in two severe forms of epilepsy.
Bloom Science plans to have its proprietary gut bacteria in the clinic for hard-to-treat patients in the next 12-18 months.

By passively collecting seizure data, Empatica's device could help patients share medical information with clinicians and make that data easier to manage.

Medical researchers frequently spend the majority of their time formatting research data and 10 percent of their time actually using it. Blackfynn hopes its platform will aid translational research and therapeutic product development.

NeuroVice's device is designed to provide a safe way to protect tongues and minimize salivation during seizures.

In a landscape where complexity has long been the norm, the power of one lies not just in unification, but in intelligence and automation.
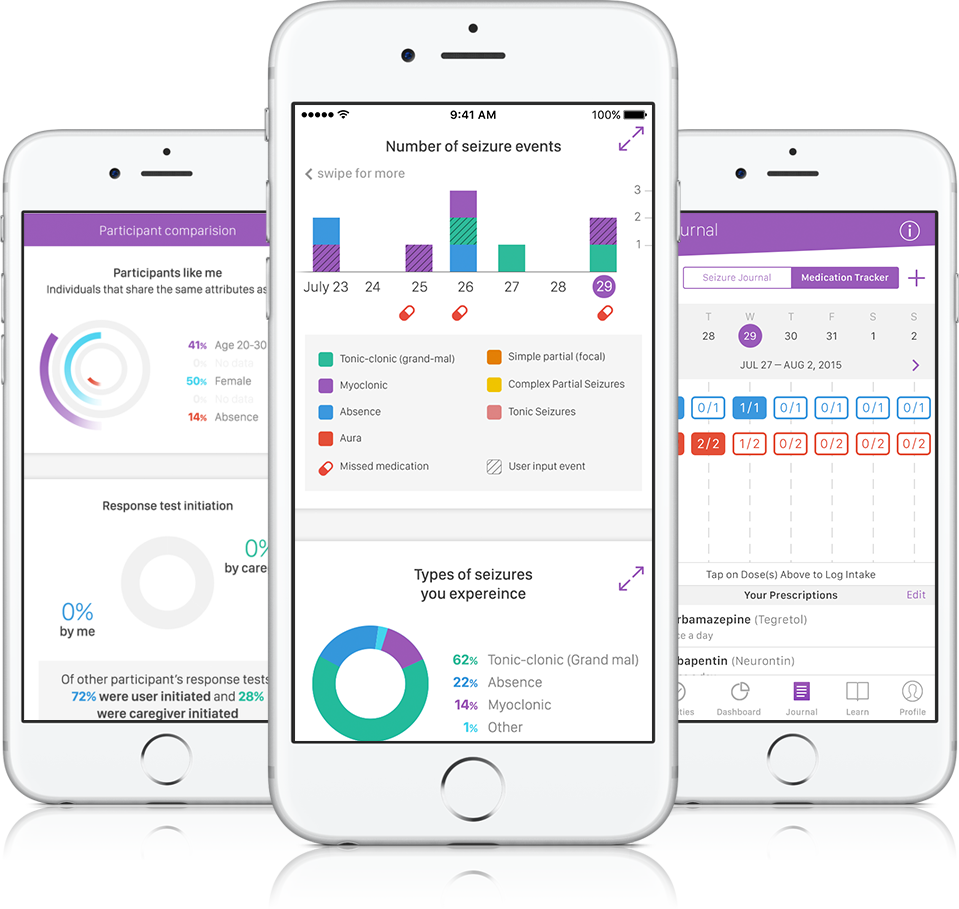
The goal is to track the severity and length of the seizure using sensors such as an accelerometer and a gyroscope as well as a memory test when the user comes out of the event.
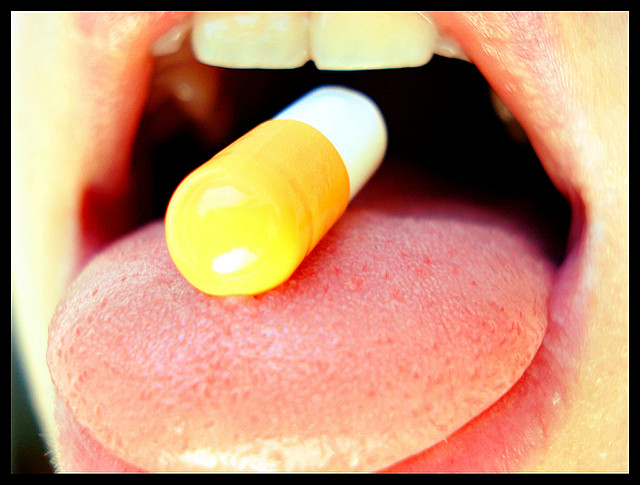
Aprecia Pharmaceuticals is getting closer to commercializing SPRITAM, its 3D printed formulation of levetiracetam, which is a well-established medication used by patients with epilepsy.

The day after news broke that Turing Pharmaceuticals' Daraprim has a new competitor on the market, CEO Martin Shkreli announced the company's gotten fast-track status for its experimental epilepsy drug.

Turing isn't just buying drugs and bumping up prices. It is actually putting a focus on new drugs, which is exemplified with its recent investigational new drug application.

The Los Angeles-based company developing its drug-resistant epilepsy system has officially backed out of its IPO plans.