
Amphivena raises $62M Series C round to develop bispecific antibody in solid tumors
The company presented data from a Phase I study in acute myeloid leukemia at the European Hematology Association's annual meeting during the summer.

The company presented data from a Phase I study in acute myeloid leukemia at the European Hematology Association's annual meeting during the summer.
The company said Wednesday that the pairing of PD-L1 inhibitor Imfinzi and CTLA-4 inhibitor tremelimumab did not prolong overall survival in previously untreated metastatic non-small cell lung cancer. The Phase III results are the latest in a string of failures for the combination.

Clever Care CEO discussed the importance of culturally-competent care.
The study, by US and Korean researchers, found Guardant360's detection of MSI-H/dMMR "highly concordant" with that of standard tissue biopsy, including in a subset of gastric cancer patients who responded to immunotherapy.

The deal includes an upfront payment and up to 100 million euros in milestones, equaling $364.5 million. Amal's lead candidate is a vaccine for colorectal cancer expected to enter the clinic later this month.
The company said the Phase III CASPIAN study of Imfinzi in first-line, extensive-stage small-cell lung cancer was successful, but did not disclose further details. Roche's Tecentriq is already approved for that indication, which accounts for two-thirds of SCLC cases.

A legal expert said such delays from CMS are unusual. The agency said May 17 that it would delay its long-awaited national coverage determination for CAR-Ts, but since then even drugmakers haven't been given a reason or timeline.

Jeff Bak shares what is needed in making healthcare more affordable and sustainable for employees.

In an interview at the ASCO meeting, BeiGene chief adviser Eric Hedrick said the company is well-prepared for when it likely regains global rights to tislelizumab.

The firm will follow a model similar to that of BridgeBio, partnering with scientific researchers and launching companies to develop and commercialize their products.
The drug was being tested in combination with radiation therapy among newly diagnosed patients with glioblastoma, a notoriously difficult-to-treat and invariably fatal disease.
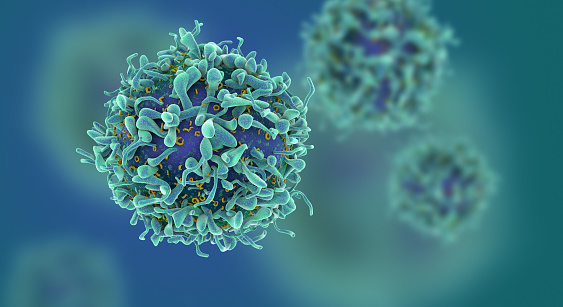
The company, part of Gilead Sciences since 2017, had placed an early emphasis on ensuring manufacturing capacity could meet demand - a key consideration in cell and gene therapy.

Verily launches a new consumer health app and the gaps it will address.

BMS's acquisition of Celgene had attracted opposition from two institutional investors. However, one of them withdrew its effort to derail the deal despite continuing to voice opposition.

The PD-L1 inhibitor is the first new drug for ES-SCLC in more than 20 years. Meanwhile, Merck is also running a Phase III study of its PD-1 inhibitor in first-line ES-SCLC.

The drug received accelerated approval for combination with Celgene's Abraxane based on Phase III data. According to a study, PD-L1-positive patients account for 26 percent of TNBC patients overall.

The deal, expected to close in the second quarter of this year, would bolster Merck's therapeutic vaccine capabilities, an analyst wrote.

The company said it had shared data from the Phase III study with the FDA. However, per FDA regulations, there is a risk that the accelerated approval for hepatocellular carcinoma could be withdrawn.