Even the most basic movements like tying a pair of shoe laces, drinking a cup of coffee or snapping a selfie can be a daunting task for someone suffering from essential tremor.
In the United States, the condition affects millions of people. Medications exist to treat the issue, but are largely ineffective for more severe cases. In those instances, the standard of care is deep brain stimulation (DBS), which involves an intensive surgery to apply electrodes near the center of the brain and connect them to an electrical pulse generator implanted in the body.The pacemaker-like device stimulates a part of the brain called the thalamus, thereby blocking the signals that cause tremors.
Imagine – as an alternative – an outpatient procedure that harnesses the power of sound and gives tremor patients back their free movement in a few hours without a single incision. Tirat Carmel, Israel-based company Insightec is making that scenario a reality with its Exablate Neuro focused ultrasound device, which uses concentrated sound waves to thermally ablate the thalamus, leading to a significant reduction in hand tremors.
Focused ultrasound works by concentrating multiple high intensity sound waves using an acoustic lens and targeting that energy source with medical imaging technology. Think of how a magnifying glass can intensify diffuse light waves into a powerful beam that can burn a hole in a sheet of paper.
Insightec was founded back in 1999, the brainchild of Kobi Vortman, a healthcare entrepreneur and expert in medical imaging. Seeded with funding from Elbit Medical Imaging and GE Healthcare, Insightec started the long road of developing and commercializing their device.
The company received FDA approval in 2004 for the use of its technology in treating uterine fibroids, followed by another approval in 2012 as a pain treatment for bone cancers and investors poured in hundreds of millions to support its growth. But, the approval and investments notwithstanding, reimbursement and a viable U.S. market for the technology failed to materialize.

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
To jumpstart a turnaround, the board appointed veteran healthcare executive Maurice Ferré as the company’s chief executive back in 2014, who refocused on Insightec’s efforts in neurology – specifically in essential tremor and Pakinson’s disease.
Prior to his position at Insightec, Ferré was the CEO of surgical robotics company MAKO Corp., and shepherded the business through its IPO and eventual $1.65 billion sale to Stryker Corp.
Insightec’s prospects improved with a raft of new financing including a $150 million Series E round led by Koch Disruptive Technologies in 2017 at a post-money valuation of $610 million. The company has raised more than $400 million.

Insightec CEO and Chairman Maurice Ferré
In a phone interview, Ferré said the treatment has enabled patients to enjoy their hobbies or extend their professional careers. One is a mother who was able to finally tie her children’s shoes, another was an artist that was able to put a paintbrush to canvas again.
“Tremor is a disease that causes a lot of problems at the social level and there hasn’t been any alternative treatment for the five million patients who are refractory to medication,” Ferre said.
Dr. Vibhor Krishna, a neurosurgeon at The Ohio State University has used Insightec’s product for more than a dozen tremor patients and said patient enthusiasm is high for an alternative to deep brain stimulation to treat their condition.
“A lot of patients have physical dependence and mental agony of living through essential tremor,” Krishna said.“The excitement has allowed these patients to at least seek out treatment and understand for themselves what would they want to do for their condition.”
There are advantages and disadvantages of focused ultrasound treatment versus deep brain stimulation. For one, deep brain stimulation can be dialed up if tremors worsen with age and dialed down in the case of side effects related to the electrical stimulation.
Focused ultrasound, on the other hand, has little risk of infection or bleeding and a much faster recovery time of hours or days, rather than weeks.
But it’s not all positive. Krishna said there is a risk in focused ultrasound of ablation spilling over into other brain structures though improvements in imaging technology is helping to lower that danger.
While clinical adoption has started to pick up, regulatory tailwinds are also pushing the company forward. So far, Exablate Neuro has treated around 2,000 tremor patients and is currently offered at 16 sites around the U.S., including top academic medical centers like UCLA, Stanford University and the Cleveland Clinic. The cost of the Exablate Neuro equipment is around $2 million and the outpatient procedure takes between two and four hours.
Insightec received FDA approval for its Exablate Neuro device to treat essential tremor in 2016. That was followed up with an expanded indication from regulators in 2018 for the treatment of with tremor-dominant Parkinson’s disease. Insightec’s pivotal study for essential tremor found that the treatment resulted in a 50 percent improvement in patient tremors and motor function scores.
Alongside the company’s regulatory wins, Insightec has finally made strides in its reimbursement strategy and is currently approved as a Medicare covered benefit in 38 states. Insightec has also won coverage from 18 Blue plans around the country, equivalent to around 50 million covered lives. More recently, the company received Japanese national reimbursement approval for Exablate Neuro.
Ferre said the reimbursement approval has helped the company usher in a growth phase with the hope to install 300 or more systems in the U.S. over the next decade.
One of the leading figures trying to establish the use of focused ultrasound in mainstream clinical practice has been Neal Kassell, the founder and chairman of the nonprofit Focused Ultrasound Foundation. A neurosurgeon by training, Kassell started the organization back in 2006.
Kassell — who was involved in commercialization efforts for the Gamma Knife focused radiation technology — was stuck by the sheer number of ways that focused ultrasound energy can be medically useful. Researchers have used focused radiation technology in use cases ranging from helping to augment immunotherapy drugs to increasing the permeability of the blood-brain barrier.
“When I saw highly-focused ultrasound I realized that this was a technology that had applications far beyond what the Gamma Knife was able to do,” said Kassell, who previously served as an Insightec board member. “It was game changing.”
A major barrier for adoption has been getting reimbursement approval for the technology from payers. Kassell said companies are often faced with the disconnect between the research needed to receive regulatory approval and evidence necessary to get the technology actually paid for in the healthcare system.
One of the reasons for the failure of Insightec’s uterine fibroids product was the status quo: the lucrative business many providers had in performing hysterectomies.
“Physicians as a group are very slow to change and resistant. And since it’s a disruptive technology, there’s fairly vicious turf battles between the different medical specialists that have to be overcome,” Kassell said.
However, there are signs of progress. According to data collected by the Focused Ultrasound Foundation, more than 100 clinical indications exist for which focused ultrasound is in various stages of research, development, and commercialization, many of which have received regulatory approval outside of the U.S. Additionally, the number of manufacturers working in the space have ballooned to nearly 50 companies.
Kassell compared the progress of focused ultrasound to that of MRI, which has seen exponential growth in clinical use over the past 20 odd years.
“Awareness is growing, but it’s still been called medicine’s best kept secret. However, that’s changing pretty rapidly,” Kassell said. “There’s regulatory approvals, insurance reimbursement is starting to kick in and by nearly every measure the field is actually starting to explode.”
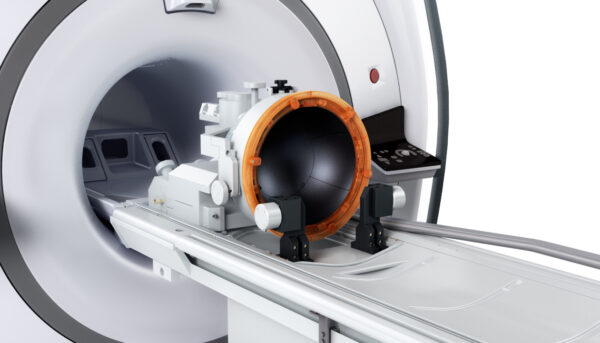
Insightec’s technology uses focused ultrasound to target structures deep in the brain.
Ferre said the company has identified seven neurological conditions to prioritize that affect a cumulative 200 million people worldwide. In the near future, Insightec is likely to tackle neuropathic pain, an indication for which it has already received approval in Europe, Russia and Korea. The company is also in Phase III clinical trials for advanced Parkinson’s disease.
Longer term though, the company hopes to expand its platform technology into areas such as targeted drug delivery, Alzheimer’s disease and epilepsy. Ferre said the company is currently running around 70 different trials around the globe at different levels of development.
It was that overarching potential that led Koch Disruptive Technologies (KDT) – the venture investment arm of the industrial conglomerate – to lead Insightec’s $150 million Series E round. It happened to be KDT’s first investment in a healthcare company.
KDT Managing Director Brett Chugg said the firm was drawn to Insightec by the pedigree of its leadership and the potential of the technology to be a safer and more cost efficient treatment option across a selection of different neurological conditions.
“We’re looking for high potential opportunities. Not individual products, but platforms,” Chugg said. “We see Insightec having that potential with a number of different indications with its ability to do everything from lesioning and ablation to helping with targeted drug delivery.”
That wide range of options provides ample open ground for development, which as Chugg puts “is not too bad of a problem to have.”
The partnership between KDT and Insightec isn’t simply a pure financial transaction. Chugg said his firm has helped broker meetings and relationships in the marketplace and and shared its expertise in areas like manufacturing.
One of the most promising applications for use of the technology is in temporarily breaking down the blood-brain barrier, which provides an obstacle for the vast majority of neurological treatments. Virtually all large-molecule neurotherapeutics and 98 percent of all small-molecule drugs are blocked by the boundary.
Dr. Krishna at Ohio State is currently running a clinical trial using the technology to explore its efficacy of using high-intensity ultrasound to open up the blood-brain barrier to potentially help treat Alzheimer’s disease by decreasing amyloid plaque load. With the use of Insightec’s technology, high intensity ultrasound waves are directed at infused micro bubbles in the bloodstream which vibrate and can make the layer permeable for around 12 to 24 hours.
Still, the application represents just one of the more than 15 different mechanisms of action for focused ultrasound that have been identified.
In sketching out his overall vision for Insightec, Ferré positions the company as part of the larger transformation of the concept of surgery from “mechanical to molecular.”
“Over the next ten years we see this MR-guided ultrasound technology as the next paradigm of surgery as we’ve gone from open to minimally invasive to robotics and now to incisionless,” Ferré said.
Picture: Insightec














