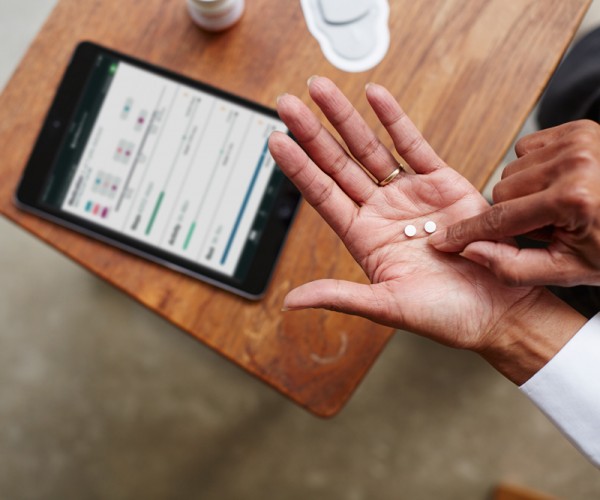
Proteus’ solution allows physicians to track medication adherence by taking a pill with a tiny sensor. The patient also wears a patch, which transmits that information to a tablet.
It’s been a rough year for Proteus Digital Health. The once high-flying medication adherence company faced staff cuts, reportedly struggled to raise funding, and now is seeing a major pharmaceutical partnership come to a premature close.
The Redwood City-based company makes tiny sensors that can be embedded in pills to track whether patients are taking their medicine. Proteus’ valuation skyrocketed to $1.5 billion three years ago, when the company gained FDA approval for Abilify MyCite, the first sensor-embedded drug. Developed in conjunction with Japan-based Otsuka Pharmaceutical Co., the digital drug was supposed to help patients with schizophrenia, bipolar disorders and depression not miss taking their medication, which can lead to serious consequences.
The two companies struck a five-year, $88 million agreement to build and commercialize a portfolio of digital health medicines for mental health. Now, Proteus will no longer work with Otsuka to commercialize these treatments.
Otuska acquired the license to develop mental health treatments using Proteus’ technology. Otsuka will no longer pay Proteus royalties going forward.
“Rather than a premature end to the agreement, it’s more of an evolution of the original agreement that allows each company to independently advance the development and commercialization of digital medicine offerings,” a spokesman for Otsuka wrote in an email. “Our digital medicine businesses have evolved to a point where we can maximize success by pursuing future opportunities independently and we are excited for both organizations moving forward.”

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
In the interim, Otuska will continue to buy sensors and wearables from Proteus. But in the longer-term, the company will look to develop digital medicines itself. Otsuka will continue to sell its Abilify MyCite System.
“As an organization, we are committed to the digital medicine business,” the spokesman wrote.
Ambitious timing
What went wrong? Michael Greeley, co-founder and general partner of Flare Capital Partners, said it likely came down to challenges in clinical workflow and pricing, along with an aggressive timeframe for commercialization.
“The 5-year business plan was in hindsight, very ambitious,” he said in a phone interview. “These are complicated products.”
He likened the contracts digital health companies sign with pharmaceutical partners to the eye-popping free agency contracts seen with professional sports teams.
“They’re super ambitious, big numbers — but there are all of these interim milestones they don’t disclose,” Greeley said. “At any point, they can restructure or terminate the agreement.”
The engineering for developing a sensor-embedded drug would be complex, not to mention the extended regulatory process, since the devices are ingested. Additionally, there’s the matter of figuring out who will handle the inflow of patient data — will time-strapped doctors be able to review it? And, perhaps most of all, there’s the consideration of cost.
The average cost of the generic for Abilify is about $500 to $800, while Abilify MyCite costs upwards of $1,600, according to GoodRx.
“They’re building at limited scale,” Greeley said. “These become quite expensive doses to deliver.”
A new focus
After Otsuka restructured its partnership with Proteus, it will shift its focus to infectious diseases and oncology. The company will also do that work with a smaller staff. A Proteus spokeswoman confirmed in an email that the company had decreased its headcount, but by a much smaller number than the 300 planned cuts it had shared with the state of California in December.
The company hadn’t disclosed any new commercial partnerships in infectious diseases or oncology yet, but it might have better luck than in its efforts with mental health medications.
A year ago, Proteus struck a collaboration with Fairview Health Services and the University of Minnesota Health to make capecitabine, a chemotherapy pill, with an embedded sensor. The drugs were prescribed to patients with stage 3 and 4 colorectal cancer, with the goal of giving oncologists insight on the best treatment regimen for each patient.
Such a sensor could keep oncologists informed when a patient is having trouble with a medication, whether because of side effects or the drug’s price. Given that most chemo drugs run for thousands of dollars — capecitabine costs anywhere from $2,800 to $4,000 — insurance companies might also have less sticker shock in paying for a dose with an embedded sensor.
Jeremy Sohn, vice president and head of digital business development for Novartis, said oncology is the right area to approach (Novartis is also an investor in Proteus).
“The concept of a smart pill, I think, is great,” he said in a phone interview. “Oncology is a space where we’ve seen really positive results from studies where more tightly integrated interactions between patients and their care community lead to better outcomes.”
He sees the value of digital medicines in helping people develop healthy habits and opening a line of communications with providers.
“If we can be thoughtful about the approaches that we take to better understand and care for patients, we absolutely should be investing in those spaces,” he said.
In the long-term, despite the recent spate of bad news, Greeley has high hopes for sensor-embedded chips. For the category to succeed, he said companies should take a more incremental approach.
“Maybe the ambitions should be more narrow,” he said, but later added:
“I can’t imagine a world in the next 10, 20 years where these products aren’t more prevalent. There’s a sense of inevitability around this category.”
Photo credit: Proteus Digital Health