The Department of Health and Human Services is easing enforcement of new interoperability rules in light of the ongoing Covid-19 pandemic.
In early March, the Centers for Medicare and Medicaid Services and the Office of the National Coordinator for Health Information Technology released two sets of rules on information blocking. They made two dramatic changes: patients would be able to more easily access their health information through apps, and health IT companies and providers could be penalized for failing to securely share health information.
CMS had originally planned to enforce the rules six months after they are published. Within that timeframe, hospitals would be required to notify a patient’s primary care provider if they were admitted to the hospital, discharged or transferred to another facility. Providers that didn’t meet these requirements would face a big penalty: they wouldn’t be able to bill for Medicare and Medicaid.
Now, CMS is giving hospitals an additional six months to come into compliance, with the new requirements going into effect in early 2021.
“Now more than ever, patients need secure access to their healthcare data. Hospitals should be doing everything in their power to ensure that patients get appropriate follow-up care,” CMS Administrator Seema Verma said in a news release. “Nevertheless, in a pandemic of this magnitude, flexibility is paramount for a healthcare system under siege by COVID-19. Our action today will provide hospitals an additional 6 months to implement the new requirements.”
Payers will also face new requirements from the rule in January of next year. Insurers offering health plans through Medicaid, Medicare Advantage and the federal exchanges will be required to implement a patient access API that lets patients view all their claims and encounter information through third-party apps. They are also required to make a similar provider directory API.

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
As for the ONC, the agency said it would exercise discretion in enforcing new requirements until three months after each compliance date. National Coordinator for Health Information Technology Dr. Don Rucker said this would provide flexibility while “ensuring the goals of the rule remain on track.”
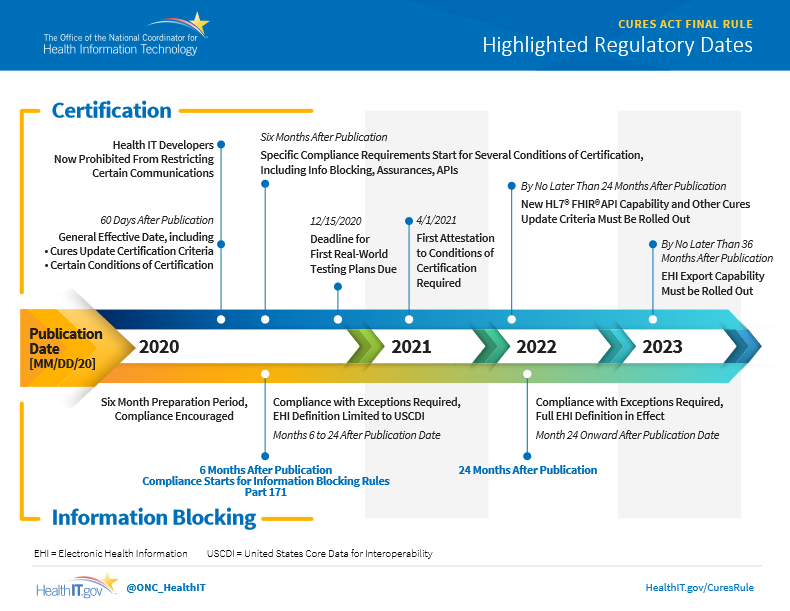
Two months after publication, the ONC’s information blocking rules will become effective, prohibiting health IT developers from restricting certain communications. Six months after the rule is published, health IT companies will be required to share electronic health information, unless exempted under the new rules.
Here’s a timeline for the ONC’s information blocking rules:
Photo credit: JamesBrey, Getty Images