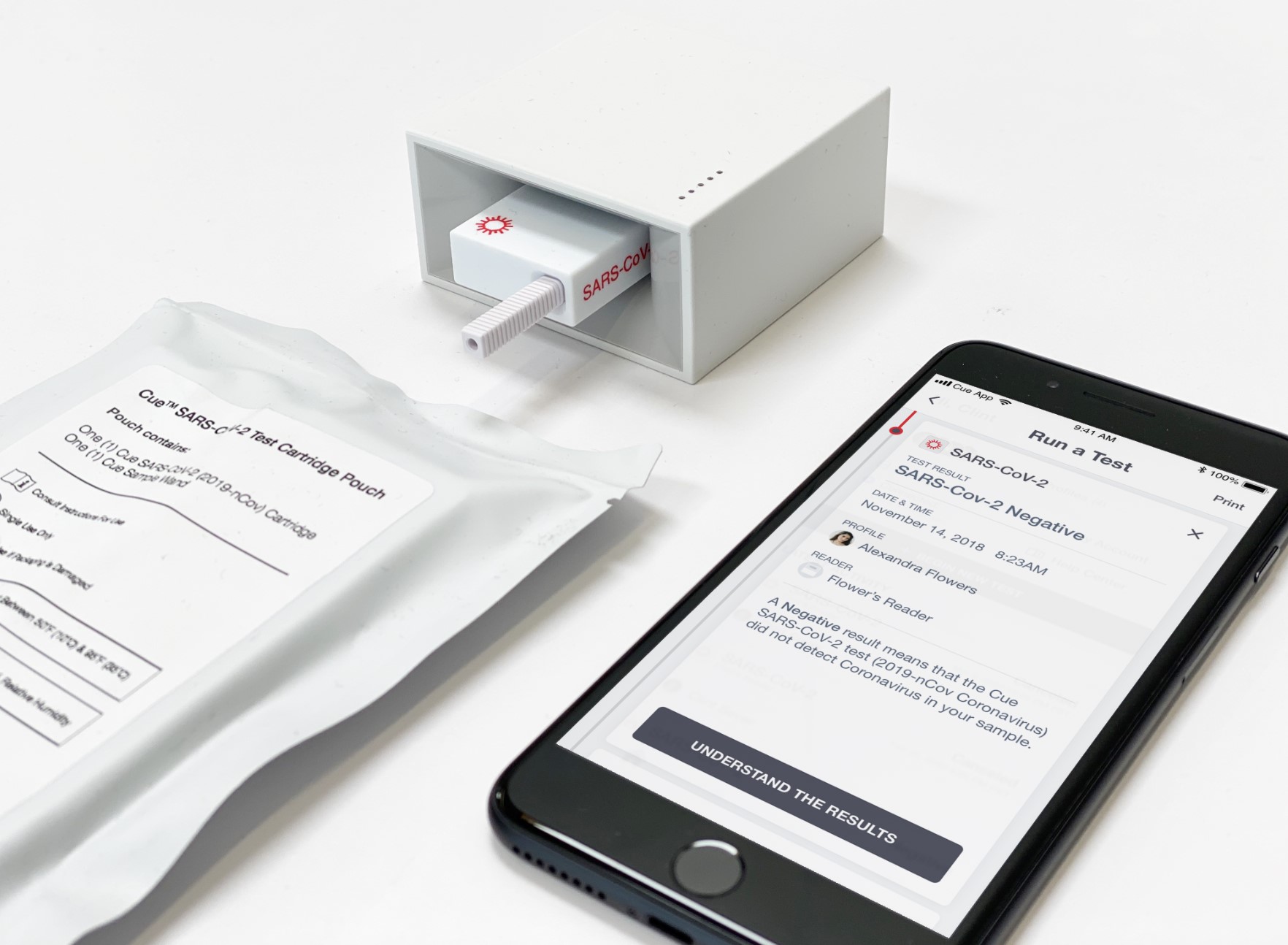
Cue Health submitted its Covid-19 test to the FDA for Emergency Use Authorization. The company is developing a portable testing platform that sends results to users’ smartphones. Photo credit: Cue Health
Cue Health, a startup developing a connected testing device, raised $100 million in funding. The San Diego-based company plans to use the funds to validate its testing platform and scale up its manufacturing facility.
The system uses disposable cartridges specific to each disease. It then sends the results to the patient’s smartphone, which they could pull up in a telemedicine consult. Cue Health currently has a 55,0000-square-foot manufacturing site in San Diego and plans to scale its facility up to more than 110,000 square feet.
Cue Health also plans to put the funds toward seeking FDA clearance for its first product, an at-home test for influenza A and B. The company is currently in validation trials that it will use for its FDA 510(k) submission. In 2018, the startup received a $30 million grant from the Biomedical Advanced Research and Development Authority (BARDA) to develop an over-the-counter flu test.
Cue Health also developed a rapid, molecular Covid-19 test that it submitted to the FDA for emergency use authorization. The test would use a nasal swab, and would be able to produce results in less than half an hour. BARDA also funded this effort, awarding a $13 million contract to Cue in March.
“The Covid-19 pandemic has highlighted the need for a rapid, easy-to-use platform for diagnostics in decentralized settings to respond to existing and emerging threats,” Cue CEO and Co-Founder Ayub Khattak said in a news release. “Healthcare settings such as nursing homes, emergency departments, and community health clinics need tools to allow them to access molecular test information immediately rather than waiting hours or days for lab results.”

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
Menlo Park-based Decheng Capital, Foresite Capital, Madrone Capital Partners, Johnson & Johnson Innovation and ACME Capital participated in Cue’s series C round. The company previously raised $45 million in 2018.
Photo credit: Abscent84, Getty Images













