While clinical trials of potential Covid-19 treatments make headline weekly, for other conditions, trials have slowed dramatically as a result of the pandemic.
In March, enrollment in new trials grinded to a halt as healthcare workers emphasized slowing the spread of the virus. Since then, at least 500 trials have resumed, according to life sciences firm GlobalData, but in a very different environment than before.
Enrollment is expected to be a challenge, with patients hesitant to attend in-person appointments. More organizations are turning to virtual and decentralized trials, with major research funders such as the U.S. National Cancer Institute allowing investigators to conduct patient assessments over video calls.
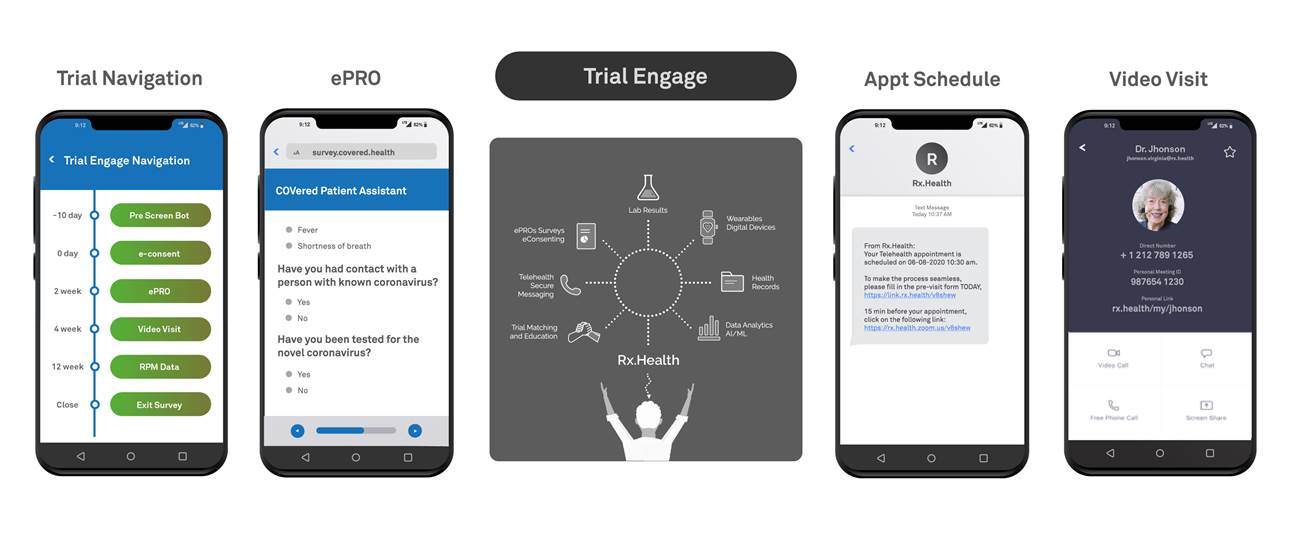
Mount Sinai spinoff Rx. Health recently put together a toolkit to help researchers improve patient engagement in virtual and decentralized clinical trials. The company is working with the American Gastroenterological Association (AGA) to create a virtual trial network, it announced on Thursday.
The set of tools helps walk patients through the registration and informed consent process. Patients can be sent wearables or other remote monitoring devices, lab results, virtual appointment reminders and access to video visits. The TrialEngage toolkit connects back into electronic health record systems and is part of Epic’s app orchard.
Dr. Chris Fourment, president of GI clinical research consortium Precision Research, said in a news release that he believed the platform would help make trial recruitment faster and more efficient.

The Funding Model for Cancer Innovation is Broken — We Can Fix It
Closing cancer health equity gaps require medical breakthroughs made possible by new funding approaches.
Ashish Atreja, co-founder of Rx.Health and chief innovation officer of medicine at Mt. Sinai’s Icahn School of Medicine, said the program was “a natural extension” of Rx.Health’s existing relationship with the AGA. They had worked together in the past to develop a system for collecting electronic patient reported outcomes for a 65-site registry.
“We started getting requests from different GI sites and most of the trials were stopped,” he said in a phone interview. “Some of those things have opened up partially. In areas where it’s getting worse — Florida and Texas right now — things are shutting down. Even if the site opens up… for research, patients aren’t that willing to take the risk right now.”
The idea was to build an expandable virtual trial toolkit that affected sites could use to jump-start trials virtually. In the future, Rx.Health hopes to expand to additional patient registries, such as cardiology and cancer networks.
Beyond the pandemic, Rx.Health still sees a use for these virtual tools. By having clinical trial participants come in for five in-person appointments per year instead of 20, more patients from a wider variety of locations would be able to participate.
“In my mind the biggest wave is going to be the post-Covid phase where digital monitoring is going to become mainstream and trial engagement is going to become mainstream,” Atreja said. “The sponsor is going to look at, why would I spend these many millions of dollars and lose this many number of participants?”
Atreja sees this as a proving point for digital health tools to show they can keep patients engaged and bring in robust trial data.
Photo credit: Rx.Health










