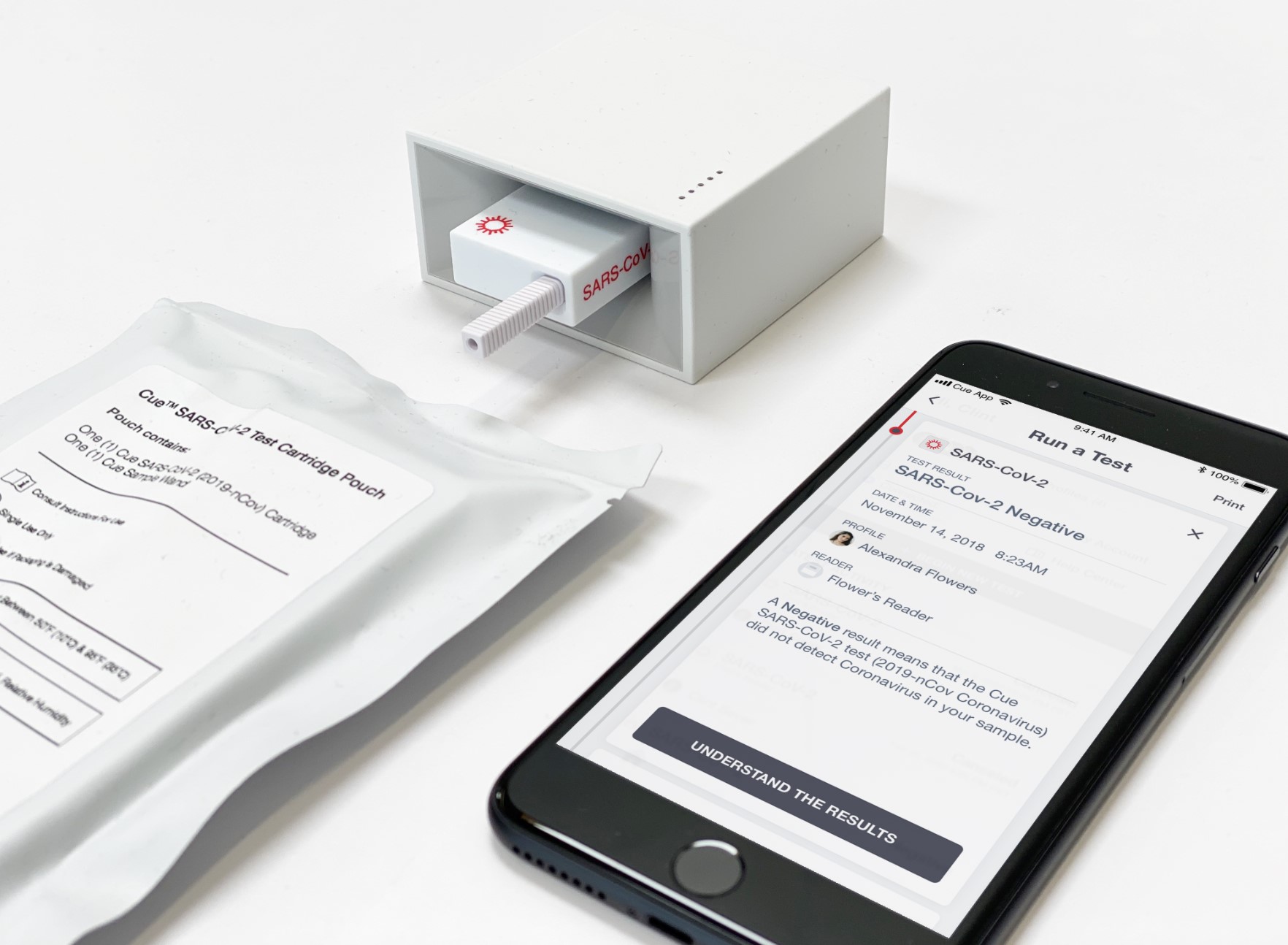
Cue Health received an emergency use authorization for its rapid coronavirus test. The company is developing a portable testing platform that sends results to users’ smartphones. Photo credit: Cue Health
At-home testing startup Cue Health raised $235 million in funding as it charts a path beyond the pandemic. The San Diego-based startup, an early provider of at-home Covid-19 tests, plans to use the funds to expand its diagnostics business.
It brought on new investors Perceptive Advisors, MSD Capital, and Koch Strategic Platforms, a subsidiary of Koch Industries, along with support from previous investors.
“The ongoing COVID-19 pandemic has exposed many of the shortfalls of the status quo healthcare model,” Cue Co-founder and CEO Ayub Khattak said in a news release. “…We believe distributed diagnostics is the key missing piece to bridge the physical to virtual care continuum, and we’re excited to have Perceptive, MSD, Koch and others join us on our mission to lead this transformation.”
Cue started in 2010 with the goal of building an at-home flu test. But after the start of the Covid-19 pandemic, it quickly found a role in providing rapid Covid-19 tests.
After receiving an emergency use authorization for its at-home test in June, Cue struck a $481 million contract with the Department of Health and Human Services and the Department of Defense to produce 100,000 test kits per day. After swabbing their nose, users insert a cartridge into its portable reader, which sends results to users’ smartphones.

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
Notably, its tests were used to screen players as part of the NBA “bubble” during the 2020 playoffs. Its tests are also being used in schools, businesses, hospitals and hotels, the company said.
More recently, it also got the greenlight to offer its test without a prescription, as part of a broader push by the FDA to expand over-the-counter testing.
In the longer term, Cue plans to expand to a broader spectrum of tests, connected to telemedicine visits.
In the meantime, the DoD and HHS are pouring funds into more startups to ramp up over-the-counter testing. Ellume, an Australian company that is also making a fully at-home test, won a $232 million contract to open a manufacturing facility in the U.S.












