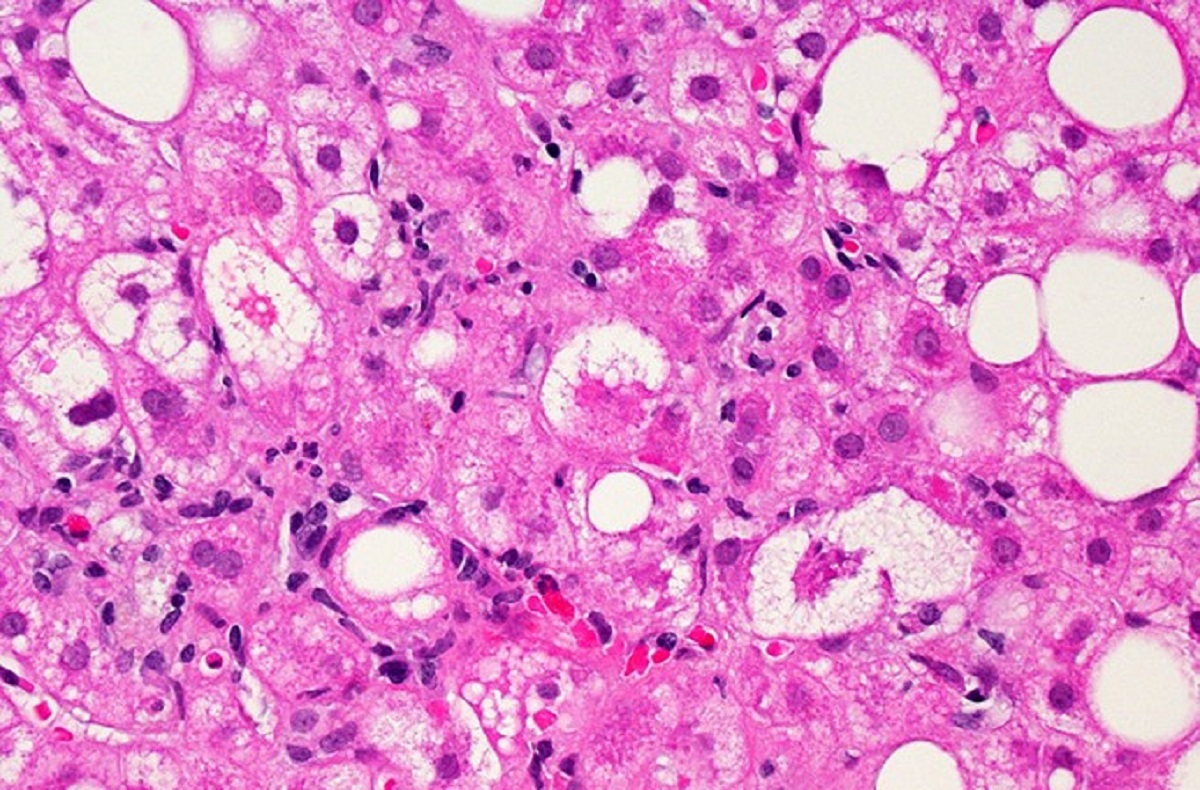
An experimental NGM Biopharmaceuticals treatment for non-alcoholic steatohepatitis, the fatty liver disease more commonly referred to as NASH, has failed in clinical testing, a surprising result considering that an earlier mid-stage study showed statistically significant patient improvement on several measures of the disease.

With the Rise of AI, What IP Disputes in Healthcare Are Likely to Emerge?
Munck Wilson Mandala Partner Greg Howison shared his perspective on some of the legal ramifications around AI, IP, connected devices and the data they generate, in response to emailed questions.
Given the clinical trial failure, NGM said Monday that the resources that had been set aside for late-stage development of the NASH drug, aldafermin, will now be applied to other internally discovered drug candidates in its pipeline, including mid-stage drug candidates for eye diseases and cancer.
NASH is a fatty liver disease that leads to scarring that damages the organ and can worsen to the point of requiring an organ transplant. The disease’s prevalence is growing due to fatty and sugary diets. The lack of any FDA-approved therapy for NASH is drawing a growing number of companies to the chase to develop one. But in the past year, several biotechs have stumbled in clinical trials and one company’s application for its NASH drug was rejected by the FDA.
NGM Bio’s aldafermin is an engineered version of fibroblast growth factor 19 (FGF19), a human hormone that plays role in controlling bile acid and also has an effect on lipid and glucose metabolism. Last August, during the digital meeting of the International Liver Congress, NGM Bio presented Phase 2 data showing that its drug led to statistically significant results in fibrosis, or liver scarring, as well as resolution of the disease.
NGM Bio had been continuing its evaluation of aldafermin in a Phase 2b study that enrolled 171 NASH patients with liver fibrosis. Those patients were randomly assigned to receive one of three doses of the study drug or a placebo. NASH severity is measured according to a scale that ranges from Stage 1, which is the lowest severity, to Stage 4, which is characterized by excessive fibrosis and loss of liver function. The main goal of the Phase 2b study was to show improvement in liver fibrosis by greater than one stage with no worsening of the disease after 24 weeks.
Despite missing the main goal of the Phase 2b study, NGM Bio executives noted that aldafermin achieved statistically significant results on secondary endpoints, including NASH resolution, which was seen at the highest of the three doses tested. The middle and high doses also met secondary goals assessing liver fat content reduction.
Nevertheless, aldafermin’s failure to achieve the main goal of the clinical trial means the drug now joins others sidelined from NASH development. Others include a small molecule developed under a partnership between Novo Nordisk and Gilead Sciences that in 2019, failed to improve fibrosis in a Phase 3 study. The R&D alliance continues with clinical testing of other drugs, alone and in various combinations.
Last May, Genfit reported its drug elafibrinor failed to beat a placebo in a late-stage NASH test. The failure sparked a corporate restructuring that shifted focus of the France-based company to another liver disorder. The failure of Genfit’s drug was followed by the FDA’s rejection of obeticholic acid, a NASH drug candidate from Intercept Pharmaceuticals.
With NGM Bio’s focus shifting from aldafermin, its only wholly owned program, the company’s drug most advanced programs are NGM621, an antibody drug candidate for the eye disorder geographic atrophy and NGM120, a potential treatment for metastatic pancreatic cancer and cachexia, which is the weight loss and wasting away caused by cancer. Both drugs are in Phase 2 testing and are covered under a partnership with Merck, according to an investor presentation.
The Merck alliance, which began in 2015, gives NGM Bio the ability to determine the scientific direction and areas of therapeutic interest with input from the pharma giant. NGM Bio is responsible for preclinical and clinical development up to human proof-of-concept studies. Merck paid NGM Bio $94 million up front and made an equity investment valued at about $106 million.
Kenilworth, New Jersey-based Merck is also contributing cash toward its partner’s R&D. Through the end of 2020, the pharma giant has paid NGM Bio $495.8 million under the collaboration, according to the biotech’s 2020 annual report. If Merck licenses any programs from the collaboration, it must pay $20 million for each.
According to NGM Bio’s first quarter financial report, the company had $425 million in total assets at the end of March, including $148.1 million in cash and cash equivalents.
Public domain image by the Flickr user NIH Image Gallery














