An Arrowhead Pharmaceuticals “gene-silencing” drug that the biotech has been quietly researching as a gout treatment will continue its development under a partnership with Horizon Therapeutics.

With the Rise of AI, What IP Disputes in Healthcare Are Likely to Emerge?
Munck Wilson Mandala Partner Greg Howison shared his perspective on some of the legal ramifications around AI, IP, connected devices and the data they generate, in response to emailed questions.
Horizon has agreed to pay $40 million up front for global rights to the drug, ARO-XDH. According to deal terms announced Monday, Pasadena, California-based Arrowhead will handle all of the preclinical research for the drug. Horizon, based in Dublin, Ireland, will be responsible for clinical development and, if approved, commercialization of the medicine. Depending on the ARO-XDH’s progress, Arrowhead could earn up to $660 million in milestone payments plus royalties from sales.
Arrowhead develops drugs that treat disease by using RNA interference (RNAi), a mechanism that stops a gene from producing a disease-causing protein. This approach is sometimes referred to as gene silencing. The RNAi mechanism is already present in cells. The company’s therapies are oligonucleotides that trigger RNAi. Arrowhead’s technology platform designs these drugs for a targeted effect that lasts for a long period of time.
The Horizon licensing deal is the latest that Arrowhead has struck for its RNAi programs. In 2016, Amgen licensed an Arrowhead RNAi drug for cardiovascular disease. Two years later, Johnson & Johnson subsidiary Janssen Pharmaceutical secured global rights to Arrowhead’s experimental RNAi drug for chronic hepatitis B virus infection. Last October, Takeda Pharmaceutical paid $300 million up front to share in the development of the biotech’s RNAi drug ARO-AAT for the rare protein deficiency called alpha-1 antitrypsin-associated liver disease.
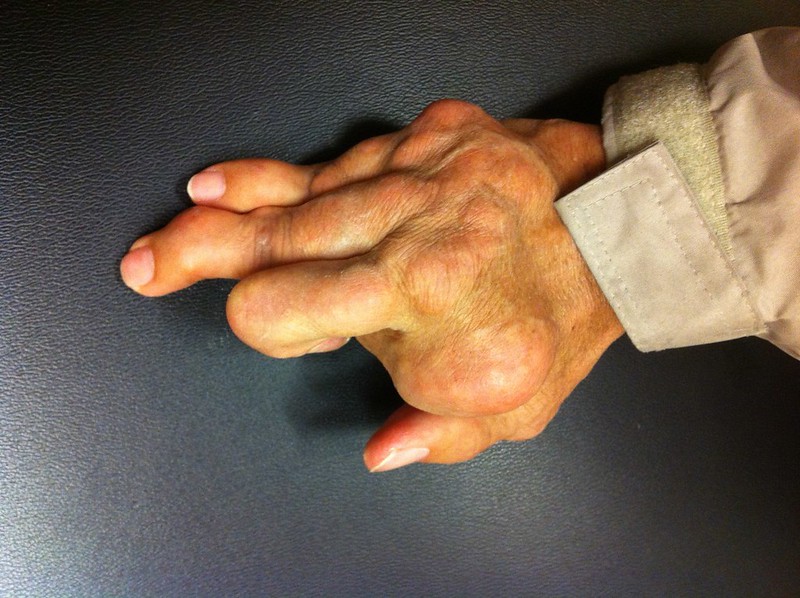
In addition to those partnered drugs, Arrowhead’s pipeline includes clinical-stage programs in liver and lung diseases, and cancer. The company’s research in gout, a serious form of arthritis caused by excess uric acid in the blood, was not disclosed until Monday’s announcement of the Horizon partnership. An estimated 9 million Americans have gout, according to the Alliance for Gout Awareness. While patients can be treated with drugs that lower uric acid levels, these drugs don’t work well enough for some patients, leaving them with ongoing swelling and joint pain in the hands, feet, and knees. Arrowhead’s ARO-XDH targets the XDH gene, which has been validated in research as the primary source of uric acid.
The first FDA approval for an RNAi drug went to Alnylam Pharmaceuticals’ Onpattro, a treatment for the nerve damage caused by the rare disorder hereditary transthyretin-mediated amyloidosis. Alnylam has since received approvals for additional RNAi drugs; the biotech could compete against Arrowhead in alpha-1 antitrypsin-associated liver disease via its partnership with Dicerna Pharmaceuticals. London-based Silence Therapeutics, partnered with AstraZeneca, is developing RNAi drugs for cardiovascular, kidney, metabolic, and respiratory disorders.
Photo by Flickr user handarmdoc via a Creative Commons license














