The field of companies pursuing a vaccine for respiratory syncytial virus (RSV) includes several pharmaceutical giants with plenty of resources and cash to pour into research. The scientists at four-year-old Icosavax believe their company’s technology could set it apart and the biotech now has $182 million for the clinical trials that could help build that case.
Icosavax’s public markets debut was the biggest life sciences IPO of last week. In addition to supporting clinical research for its lead program in RSV, the company is also moving toward clinical testing of a vaccine that may be able to address variants of the novel coronavirus.
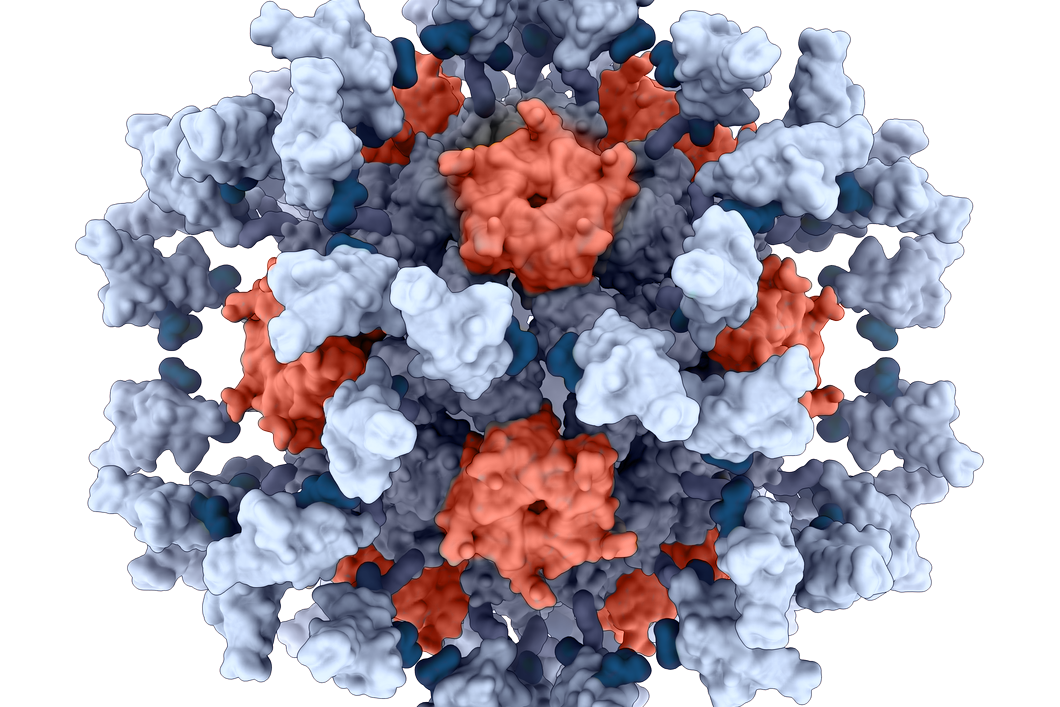
The vaccines of Seattle-based Icosavax are made with proteins that the company designs and engineers to resemble viruses. These virus-like particles (VLPs) are intended to elicit an immune response, but because they contain none of the genetic material of a virus they cannot replicate and cause infection. And RSV infection can become severe, particularly for young children and the elderly. According to the Centers for Disease Control and Prevention, RSV leads to about 58,000 annual hospitalizations of children age 5 and younger. In adults 65 and older, RSV infection leads to about 177,000 hospitalizations and 14,000 deaths.
Icosavax’s RSV program, IVX-121, has reached Phase 1 testing in RSV. Companies that are further along in clinical development with RSV vaccines include GlaxoSmithKline, Pfizer, Moderna, Janssen, and Meissa Vaccines. But Icosavax says that one of the features of the VLP technology is the ability to produce a vaccine with the capacity to address multiple pathogens. The company is pairing its RSV vaccine candidate with a VLP for human metapneumovirus (hMPV), another dangerous respiratory virus that also has no available vaccine. This bivalent vaccine candidate, which the company has named IVX-A12, is being readied for clinical testing.
“We believe the induction of nAbs (neutralizing antibodies) is key for both RSV and hMPV vaccine efficacy in older adults and that multivalent VLP display of the prefusion RSV and hMPV antigens on our VLP candidates will induce a stronger nAb response than other vaccine technologies,” the company said in the IPO filing.

Transforming the OR: CEO Reveals Game-Changing AI Tech for Better Efficiency
How Apella leverages technology to increase OR efficiency.
IVX-A12 is intended to protect the elderly, who are more vulnerable, as RSV and hMPV are both common causes of pneumonia in older adults, the company explained in the filing. A clinical trial evaluating this vaccine is planned to start in Belgium in the second half of this year; preliminary data are expected in the first half of next year.
Depending on the clinical trial results of the bivalent vaccine candidate, Icosavax expects it could file an investigational new drug application with the FDA in the first half of next year. That Phase 1 clinical study is designed to enroll about 100 healthy adults who will be followed for seven months. The vaccine will test multiple doses with and without an adjuvant, an ingredient that boosts the immune response. This design will enable the company to evaluate immune responses to the individual components of the vaccine, the filing stated.
During the pandemic, Icosavax used its VLP platform to develop vaccine candidates addressing SARS-CoV-2. That research has produced two vaccine candidates. Lead candidate IVX-411 incorporates the ACE2 receptor binding domain (RBD) from the spike protein of the original virus. In results of preclinical research that were published last October in the journal Cell, this vaccine was able to elicit neutralizing antibodies that offered protection against the novel coronavirus. Icosavax began a Phase 1/2 study in Australia in June. Proof of concept data are expected in the first half of next year.
In April, Icosavax closed a $100 million Series B round of financing. CEO Adam Simpson told MedCity News at that time that VLP vaccines offer a breadth of response that could apply to variants of the novel coronavirus. The company’s second coronavirus candidate, IVX-421, incorporates an RBD protein with what Icosavax describes as “critical mutations” from the beta strain of SARS-CoV-2. Research on this candidate is preclinical; the company plans to test whether IVX-421 is able to induce a stronger immune response against the original virus and the beta strains compared to IVX-411. Depending on results of tests of both vaccines the company said it may incorporate IVX-421 into the clinical trial plans.
According to the IPO filing, Icosavax has budgeted $120 million for developing IVX-A12 through Phase 2b testing. About $35 million will go toward the development of other vaccines, including IVX-411, which the company plans to advance through the completion of Phase 1/2 and Phase 2 clinical trials. Icosavax estimates that its cash holdings combined with the IPO proceeds will last through at least 2024.
Image by Icosavax








