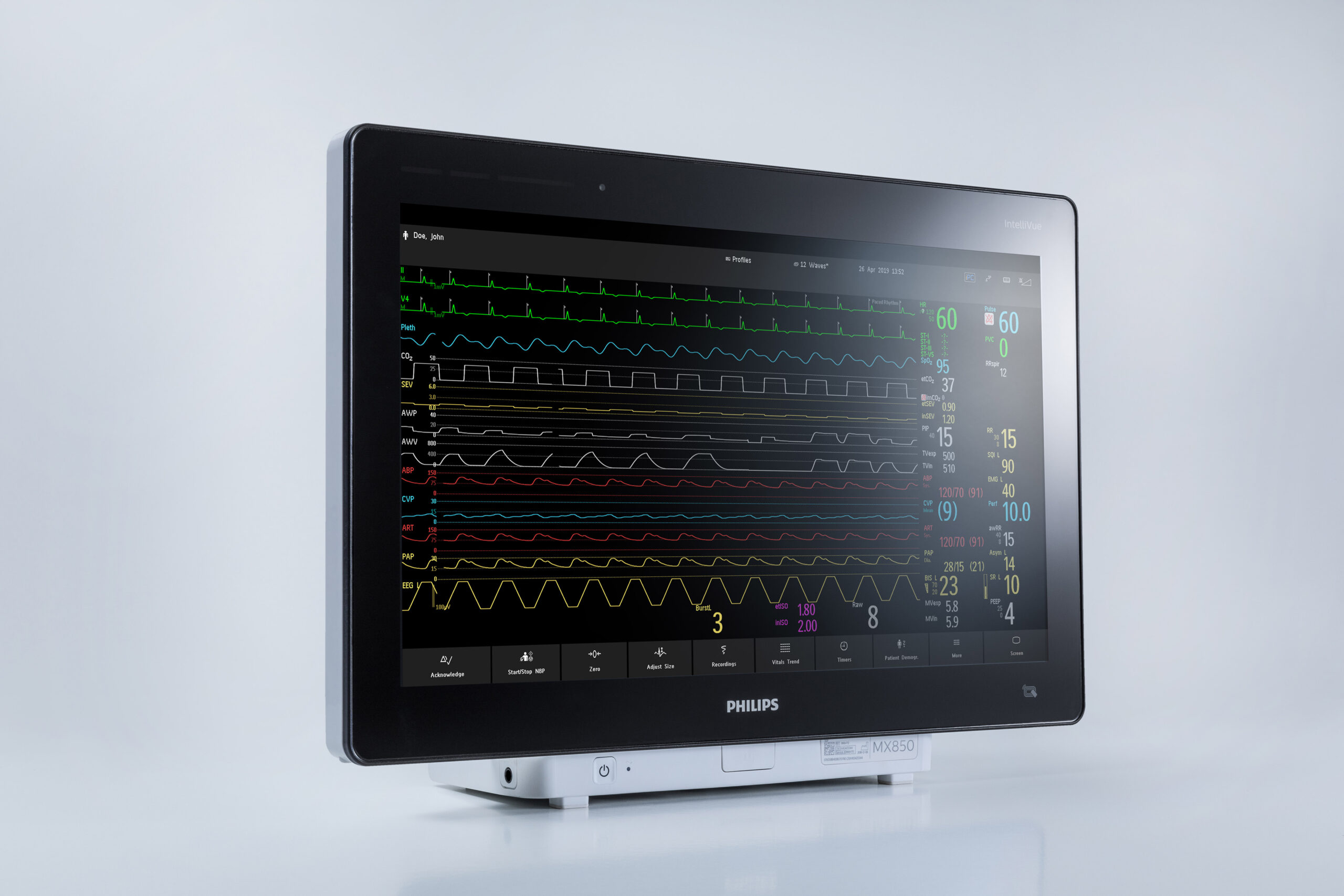
An IntelliVue MX850 monitor displays patient’s vitals. Philips recently received FDA 510(k) clearance for its IntelliVue MX750 and MX850 devices. Photo credit: Philips
A year after they were given an emergency nod to be used during the pandemic, two of Royal Philips’ newest patient monitors now are cleared by the Food and Drug Administration. The agency recently granted 510(k) clearance to Philips’ IntelliVue MX750 and MX850, which are designed to provide real-time information on a patient’s vitals and support remote monitoring in a hospital setting.
The FDA initially gave Philips an emergency use authorization for the devices in April of last year, shortly after the start of the Covid-19 pandemic. Given that the devices could be used to remotely monitor hospitalized patients, the intent was to use them to watch hospitalized Covid-19 patients’ vitals without exposing healthcare workers to the virus during a time where masks and other protective equipment were scarce.
Philips says the devices feed encrypted patient data into a hospital’s EMR, offering a real-time view of their conditions. They can connect to devices, such as IV pumps, anesthesia machines and ventilators. The monitors can also support clinical decision support tools, such as one that Philips developed to show changes in vital signs and provide context about a patient’s condition.
The FDA clearance just includes the two monitors, but add-on display screens and a module server rack were also included in the emergency use authorization. The full system has been used in Europe since it received a CE mark in 2019.












