Bristol Myers Squibb was the first company to win FDA approval for a CAR T-cell therapy for multiple myeloma, and Johnson & Johnson could soon become the second. Arcellx has some catching up to do but its technology could offer some key advantages compared to the first-generation CAR T-therapies of its larger rivals. The biotech has encouraging early clinical data for its multiple myeloma CAR T, and it now has $123.8 million in IPO cash to advance that therapeutic candidate to a pivotal clinical trial.
Gaithersburg, Maryland-based Arcellx priced its offering of 8.25 million shares at $15 apiece, which was the low end of its planned $15 to $17 per share price range. Those shares began trading on the Nasdaq Friday under the stock symbol “ACLX.” Arcellx’s stock price opened at $19 before settling down to $16.80 at the market close.
The “CAR” part of CAR T stands for chimeric antigen receptor—the receptor that recognizes and binds to an antigen on a cancer cell. CAR T-cell therapies are made by harvesting a patient’s T cells and genetically engineering them to express antigen-specific CARs on the cell surface. The first CAR T therapies from Novartis and Gilead Sciences won FDA approvals for certain types of blood cancer that had not responded to earlier therapies. BMS’s CAR T-therapy, Abecma, was approved for multiple myeloma last year.
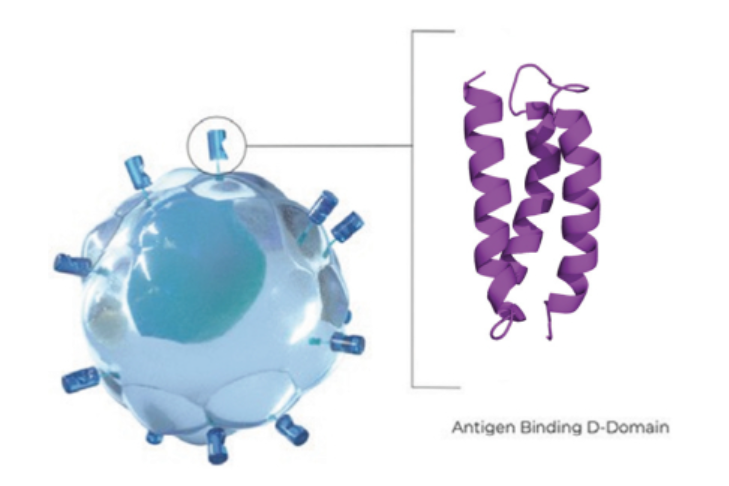
While CAR T offers a way to address blood cancer cases that have failed earlier lines of treatment, some patients do not respond to these therapies, or they relapse later. CAR T also introduces the risk of dangerous side effects. Arcellx aims to overcome those challenges with technology that enables it to produce synthetic binding scaffolds that are “deimmunized,” which is the company’s way of saying that they are engineered to avoid causing an immune response.
The synthetic binding scaffolds, which Arcellx calls D-Domains, are smaller than the binding domains in currently available CAR T therapies. In its IPO filing, Arcellx said that the smaller size allows it to reduce the size of the lentivirus construct used to deliver its genetic cargo. Consequently, these therapies may have better transduction, which is the transfer of the genetic material into a T cell. In preclinical research, Arcellx said D-Domain led to higher transduction efficiency, a higher expression of its binders on the surface of T cells, and lower signaling that can lead to off-target activation of the T cells.

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
Arcellx’s lead program, CART-ddBCMA, is a CAR T-therapy designed to target BCMA, a protein found in abundance on the surface of multiple myeloma cells. The company noted in the IPO filing that it has achieved transduction efficiency higher than published Phase 1 data for BMS’s Abecma and Johnson & Johnson’s experimental CAR T therapy, ciltacabtagene autoleucel, both of which target also BCMA. Arcellx’s comparison comes with the caveat that it is looking across trials with different designs and methodologies, rather than head-to-head studies. Nevertheless, more efficient transduction is one sign that the Arcellx drug could be competitive to BMS’s Abecma and J&J’s CAR T, which is expected to receive an FDA decision by the end of this month.
At the 2021 annual meeting of the American Society of Hematology in December, Arcellx presented data for the first 24 multiple myeloma patients dosed with CART-ddBCMA in a Phase 1 expansion study. Of those patients, 22 could be evaluated for safety, 17 of whom had disease that was unresponsive to five earlier lines of treatment. As of the Nov. 4, 2021 cutoff date, 19 patients could be evaluated for efficacy. Arcellx reported an overall response rate of 100% in all 19 and a complete response or a stringent complete response in 68% (13 of 19) patients. The median follow-up period for these patients was 283 days.
CAR T therapy’s side effect risks include cytokine release syndrome and neurotoxicity. Arcellx reported that these problems were manageable and resolved with standard treatment measures at the two dose levels tested. No off-target effects were observed. Arcellx now plans to advance CART-ddBCMA to a pivotal 100-patient Phase 2 clinical trial by the end of 2022. In the IPO filing, the company said that based on its discussions with the FDA, successful data from this study combined with the Phase 1 results should be enough to support a biological license application submission. The company also hopes expansion studies from the Phase 2 test will support use of CART-ddBCMA as an earlier line of treatment. For context, Abecma’s approval covers its use treating multiple myeloma unresponsive to at least three earlier lines of treatment.
The synthetic binding domains Arcellx produces are key components of a second platform technology that the company calls ARC-SparX. This technology produces CAR T-cell therapies engineered to target multiple antigens in order to adapt as a patient’s cancer changes. Also, the dose of these therapies can be controlled in order to minimize toxic effects. The most advanced drug from this platform, ACLX-001, is on track to enter Phase 1 testing as a treatment for relapsed or refractory multiple myeloma; ACLX-002 is being developed for acute myeloid leukemia and high-risk myelodysplastic syndrome.
The potential competitive advantages of Arcellx’s approach could go beyond blood cancers. CAR T-therapies have not been able to hit solid tumors. Arcellx contends its technology produces binders that do. The tech can also be applied to off-the-shelf cell therapies. These applications are not limited to T cells. Natural killer cells are among the other cell types listed by the company for potential use of D-Domain. Longer term, the company is looking outside of cancer.
“Ultimately, we hope to develop multiple therapeutic options incorporating different mechanisms, cell modalities, and targets to treat hematologic cancers, solid tumors, and indications outside of oncology, such as autoimmune diseases,” Arcellx said in its filing.
Arcellx was founded in 2014. Prior to the IPO, Arcellx had raised $234.8 million in financing, most recently a $115 million Series C round of funding that closed last April. New Enterprise Associates is the biotech’s largest shareholder owning a nearly 14.5% post-IPO stake, according to the filing. SR One owns 11.4%; Novo Holdings owns 9.5%.
As of the end of the third quarter of 2021, Arcellx reported its cash position was $131.2 million. That money, combined with the IPO proceeds, will be deployed across the biotech’s pipeline. Between $75 million and $85 million is budgeted for the Phase 2 test of CART-ddBCMA. Another $10 million to $20 million is earmarked for drug candidates from the company’s ARC-SparX platform.
Image from Arcellx IPO prospectus













