Miach Orthopaedics — a medical device company focused on restoring torn anterior cruciate ligaments (ACLs) instead of reconstructing them — received $40 million in funding on Wednesday.
The financing includes $30 million in investments from Sectoral Asset Management, Endeavour Vision, NFL Players Association, Amzak Health, Smith+Nephew and DSM Venturing. The Massachusetts-based company also signed a $10 million venture debt term sheet with Silicon Valley Bank.

With the Rise of AI, What IP Disputes in Healthcare Are Likely to Emerge?
Munck Wilson Mandala Partner Greg Howison shared his perspective on some of the legal ramifications around AI, IP, connected devices and the data they generate, in response to emailed questions.
This funding round will help expand the U.S. commercial rollout of Miach’s lead product — the Bridge-Enhanced ACL Restoration (BEAR) implant. The company was founded in 2016, but work on the BEAR implant began “many years earlier,” said CEO Patrick McBrayer.
The implant’s development was pioneered by Miach’s founder, Dr. Martha Murray, at Boston Children’s Hospital’s orthopedic surgery department. McBrayer said that Dr. Murray’s mother suggested the company’s name — Miach was a healer in Irish mythology who helped a king regenerate his arm after he lost it in battle.
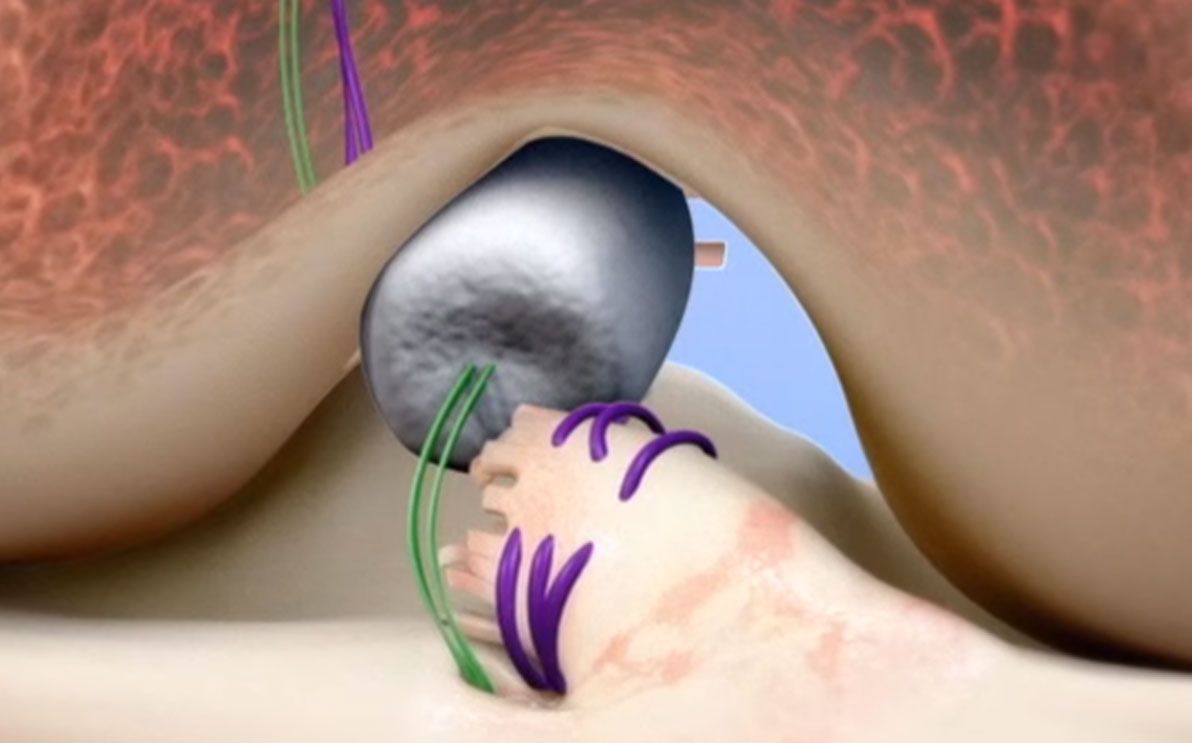
The BEAR device acts as a bridge between the two ends of the torn ACL. When conducting the minimally invasive procedure, a surgeon injects a small amount of a patient’s own blood into the implant and inserts it between the torn ends of the ACL.
The combination of the implant and the patient’s blood helps the body heal ACL tears while maintaining the ligament’s original attachments to the femur and tibia. The body reabsorbs the implant as the ACL healing process goes on.
This procedure is much more favorable than the standard of care for ACL tears — surgical reconstruction — for a few reasons, McBrayer declared. First, the BEAR implant restores the native ACL in its original anatomical location. He also pointed out that implanting the BEAR device does not require a second surgical wound site to remove a healthy tendon from another part of the body, nor does it require the use of a deceased donor’s tendon. The BEAR implant also allows for faster recovery of muscle strength, McBrayer said
Without treatment, ACL tears cannot heal — that’s why about 400,000 ACL reconstruction surgeries are performed each year in the U.S.
ACL reconstruction surgery is one of the most common orthopedic procedures in the country, but McBrayer argued that we must move away from this standard of care. Reconstruction surgery often results in a re-tear, and a large number of athletes who undergo this procedure aren’t able to return to the same level of sport at which they once played.
“Being able to repair a full-thickness ACL tear has been the holy grail of sports medicine for quite some time,” McBrayer said. “The BEAR implant is the first of its kind, as evidenced by the de novo FDA approval.”
The device received its de novo clearance in 2020. It is indicated for skeletally mature patients who are at least 14 years old, have a complete rupture of the ACL confirmed by magnetic resonance imaging, and have an ACL stump attached to the tibia. The device must be implanted within 50 days of the ACL tear.
More than 500 patients have been implanted with the BEAR device since its commercial rollout in the fall of 2021. More than 150 surgeons in 33 states have performed the procedure, McBrayer said.
Photo: Miach Orthopaedics














