
Medtech companies are evolving rapidly as more and more of them develop products that collect and leverage substantial patient and provider data.
Companies that once only developed hardware-based solutions for medical problems are now evolving into data platform companies, offering a more comprehensive glimpse into the habits and health of their patients and customers. Many of these solutions leverage artificial intelligence (AI) and machine learning (ML), for which intellectual property tends to be more challenging to protect through traditional approaches.

Health Benefit Consultants, Share Your Expert Insights in Our Survey
Kristin Havranek, Martin Gomez, Matt Wetzel, Steven Tjoe and Stephanie Philbin Kristin Havranek has worked extensively with and in the MedTech industry for the last 25 years. A trusted advisor to investors and companies alike, Ms. Havranek regularly helps startup and emerging MedTech companies build their IP portfolios in a strategic, thoughtful manner with their […]
Investor and strategic partner mindsets have shifted toward optimal ways to protect their intellectual property (IP) requirements, especially given evolving laws and social concerns.
Having had decades of experience working with medtech companies, we have identified the top 5 IP considerations they should be aware of in their product development lifecycle. This is especially true for medtech-enabled data platforms
Medtech companies should consider the following IP protection strategies in the emerging fields of software as a medical service (SaMD), software in a medical device (SiMD), and AI in medical technologies.
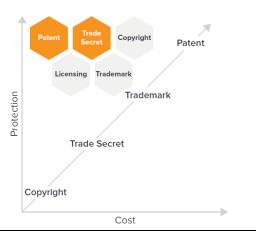
1. How to strategically diversify IP protection

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
-
-
- Patent the device and consider patenting the software it utilizes.
- Alternatively, or additionally, consider protection of methods, AI/ML mechanisms and software as a trade secret.
- Ensure non-compete and confidentiality agreements with employees are in place, to the extent possible given jurisdictional constraints.
- Consider exclusive licensing of training/learning databases to exclude others from developing similar solutions using these databases.
- Albeit narrower protection, copyright and trademark as appropriate.
-
2. Whether to patent or keep innovations as trade secret
Generally speaking, the ability to reverse engineer is the key inquiry in deciding if patent or trade secret is the appropriate mode of protection for a company’s data-enabled invention, along with the evolving laws around eligibility of patenting of such inventions (See IP Point 4, below).
-
-
- Patenting must reveal details, which can be challenging when protecting data-enabled software that includes AI/ML aspects, broadly. Thus, patenting is best for devices and for protecting the interplay of physical products and software.
- Trade secret requires you to maintain and protect secrecy indefinitely, but can be imitated if your idea garners attention. It is often best for software, manufacturing methods, or products that are costly or difficult to imitate.
-
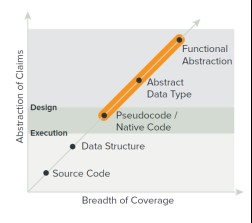
3. If patenting, how to balance breadth and abstraction in patent claims to maximize protection
Claiming software uses necessary functional language to describe the invention… which can be too abstract for protection by patent law if at too high of a level. Nevertheless, patentees will always seek to broadly cover their invention, thus resulting in the tension that exists between breadth and abstraction levels in software patent drafting.
*What is Functional Language? – Functional language explains what the invention does rather than what it is.
-
-
- Strike a balance in the claims between permissible breadth and amount of abstraction in the claim. Functional abstraction through pseudocode/native code should be covered in varying claim structures.
- Be more detailed in the specification. Draft full descriptions of examples and use pictures to depict examples and describe both the functional abstraction level of detail through the pseudocode/native code in the specification.
-
4. Keeping up with the evolution of subject matter eligibility in patents
U.S. law does not allow patenting of abstract ideas** or laws of nature.*** In 2014, the U.S. Supreme Court broadened this concept, giving the U.S. Patent Office additional ways to reject applications for AI-based patents and for trial courts to invalidate them. This decision has made it more challenging to obtain software and AI-based patents in the United States. There are creative ways to use the evolving case law and to present inventions to fall outside of this prohibition, but it is important to remain diligent of the volatile state of the industry.
**Abstract ideas include (i) mathematical concepts, (ii) methods of organizing human activity and (iii) mental processes.
***Laws of nature include natural phenomena and products of nature;a discovery of something that is a natural law, not an invention.
5. Artificial intelligence considerations: Permission, assignments, copyleft and open source
-
-
- Ensure you have permission to use the data.
- Consider inventorship and get assignments from individuals who: (i) select the data acted upon by AI, (ii) review results or outputs of an AI engine, (iii) select ML algorithms used to train an AI model, and/or write the source code to implement AI.
- Be careful about open source usage and avoid copyleft.
-
Photo: stocknshares, Getty Images
Kristin Havranek has worked extensively with and in the MedTech industry for the last 25 years. A trusted advisor to investors and companies alike, Ms. Havranek regularly helps startup and emerging MedTech companies build their IP portfolios in a strategic, thoughtful manner with their business goals and needs in mind. She evaluates freedom-to-operate, patentability, investor due diligence and financing IP, and conducts patent validity analyses. She advises on strategic alliances and prepares and prosecutes patent applications before the U.S. Patent and Trademark Office. Ms. Havranek’s ties in the industry run deep and is currently on the leadership team of MedtechWomen.
Martin Gomez is a partner in Goodwin’s Technology Companies group specializing in intellectual property matters. Mr. Gomez focuses his practice on advising technology and life sciences companies of all sizes (including startups), and their investors, in corporate and especially intellectual property matters throughout the business life cycle, including new company formation, IP protection, fundraising, strategic transactions and exits.
Matt Wetzel is an experienced health lawyer and provides strategic advice to life sciences companies on complex health care laws and regulations, including federal and state fraud and abuse laws, Medicare and third party billing and reimbursement regulations, patient privacy obligations, and transparency requirements, among other areas. Matt also works with clients to establish and operate effective global compliance and risk management programs.
Steven Tjoe is a partner in the firm’s Technology and Life Sciences groups and a member of the firm’s FDA practice. He focuses his practice on product development strategies and regulatory compliance counseling, in particular as related to medical devices, digital health products, in vitro diagnostics, laboratory developed tests, compounded drugs, cell and gene therapies, and other drugs and biologics. Steven advises clients in analyzing premarket pathways, product adverse event risk profiles, product communications and marketing, and GMP compliance. Steven also advises on Hatch-Waxman patent listing and exclusivity issues, is a contributor to Goodwin’s Guide to Biosimilars Litigation and Regulation in the U.S, and regularly conducts risk analyses for offerings and transactions involving FDA-regulated entities across the medical device, drug, and biologic industries.
Stephanie Philbin, a partner in the firm’s FDA practice and a member of its Life Sciences group, concentrates on Food and Drug Administration laws. She joined Goodwin in 2007.












