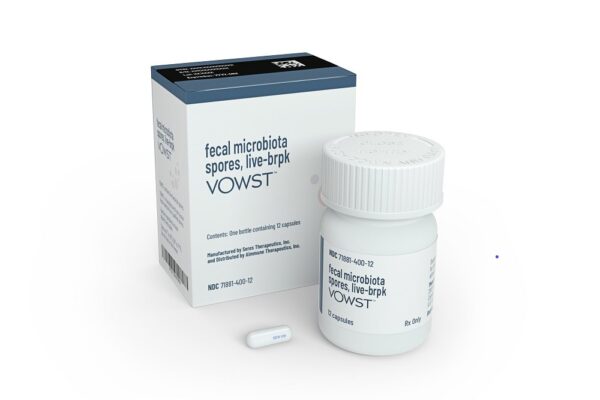
A capsule filled with bacteria is now FDA approved for treating a serious and potentially deadly type of gut infection, making the Seres Therapeutics drug the first oral microbiome therapy to receive the regulatory go-ahead for patients.
The infection is caused by Clostridioides difficile (C. difficile or C. diff), a bacterium that’s part of the diverse microbial community in the intestines. When the gut microbiome is disrupted, C. diff can proliferate, releasing toxins that cause frequent diarrhea, abdominal pain, and fever. In severe cases, the infection leads to organ failure and death. Antibiotics can treat C. diff infection, but these bacteria can develop resistance, leading to a recurrence of the infection. When that happens, patients have limited treatment options.

Behavioral Health, Interoperability and eConsent: Meeting the Demands of CMS Final Rule Compliance
In a webinar on April 16 at 1pm ET, Aneesh Chopra will moderate a discussion with executives from DocuSign, Velatura, and behavioral health providers on eConsent, health information exchange and compliance with the CMS Final Rule on interoperability.
According to Centers for Disease Control and Prevention data cited by Seres, about 156,000 cases of recurrent C. diff infections are reported in the U.S. annually, and more than 20,000 of these infections lead to death. The FDA approved Vowst for preventing the recurrence of C. diff infection in adults who have already been treated with antibiotics.
“The approval of Vowst is an incredible moment for Seres, and we think it’s just the beginning of what is possible with microbiome therapeutics,” CEO Eric Shaff said, speaking during a Thursday conference call. “We believe our microbiome approach has the potential to address a range of infectious diseases in other serious indications with new therapeutic options.”
Rather than kill off C. diff, the Seres drug is intended to restore balance to the gut microbiome. The idea of using bacteria as a therapy came from fecal microbiota transplants (FMT), in which stool from a healthy donor is administered into the intestine of a patient. These procedures have been done for years with little FDA oversight and while they can work, they also introduce the risk of transmitting dangerous pathogens.
Last November, FDA approval of a Ferring Pharmaceuticals’ FMT treatment for recurrent C. diff infection made it the first approved fecal microbiota treatment. The product, named Rebyota, consists of donor stool that has been tested for known pathogens. Stored frozen, the product must be thawed and administered by a healthcare professional in a clinical setting.

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
In most medicines, the therapeutic benefit comes from an active pharmaceutical ingredient, such as a small molecule designed to hit a target in the body. For the Seres drug, the key ingredient is more than active. It’s alive. Like the Ferring treatment, Seres’s Vowst is also made from stool of healthy donors. But Seres’s manufacturing process screens the stool for pathogens, then processes the source material to isolate and purify spores of Firmicutes, a type of beneficial bacteria. Vowst is a capsule that can be kept at room temperature and taken by the patient at home. The dosing regimen is four capsules taken once a day for three consecutive days.
Seres was formed in 2010 by venture capital firm Flagship Pioneering, making it one of the first microbiome startups. Its C. diff therapy, known in development as SER-109, was the biotech’s lead program and its ups and downs were often viewed as a bellwether for the emerging microbiome therapy field. In 2016, Seres reported its C. diff therapy failed a Phase 2 clinical trial, results that sank the biotech’s stock price and sent chills throughout the microbiome sector.
Seres’s reanalysis of the data found that some of the patients in the study did not actually have C. diff infection. The company also concluded that it did not use a high enough dose. With FDA permission, the company resumed clinical testing.
The FDA based its approval on Phase 3 results showing Vowst reduced the rate of recurrence of C. diff infection. After eight weeks, the infection rate was 12.8% in the Vowst group compared with 39.8% in the placebo group. The most commonly reported side effects included abdominal bloating, fatigue, constipation, chills, and diarrhea. The results were published last year in the New England Journal of Medicine.
Recurrent C. diff infection has become a microbiome drug proving ground, attracting the research efforts of several companies. Finch Therapeutics reached Phase 3 testing with its experimental therapy oral containing a diverse community of microbes intended to restore balance to the gut microbiome. Early this year, Finch stopped the program, citing study enrollment difficulties and financial challenges. Vendanta Biosciences is still in the chase with VE303, an oral therapy consisting of selected bacteria that together are intended to offer a therapeutic effect. On Tuesday, Vedanta announced the close of $106.5 million in financing to advance its oral C. diff therapy to Phase 3 testing.
Vowst carries a wholesale price of $17,500 per course of therapy. Seres will commercialize its new product under a partnership with Nestlé Health Science. According to the agreement struck up in 2021, the two companies will share equally in the profits of the drug. Seres expects the product will become available in June. With the approval, Seres is set to receive a $125 million milestone payment from Nestlé.
The next microbiome therapy in the Seres pipeline is SER-155, a therapy comprised of a consortia of bacteria. This oral treatment is being developed to reduce gut infections, bloodstream infections, and graft versus host disease in cancer patients undergoing hematopoietic stem cell transplants. Seres expects preliminary data from a Phase 1b study will become available next month.
Photo by Seres Therapeutics