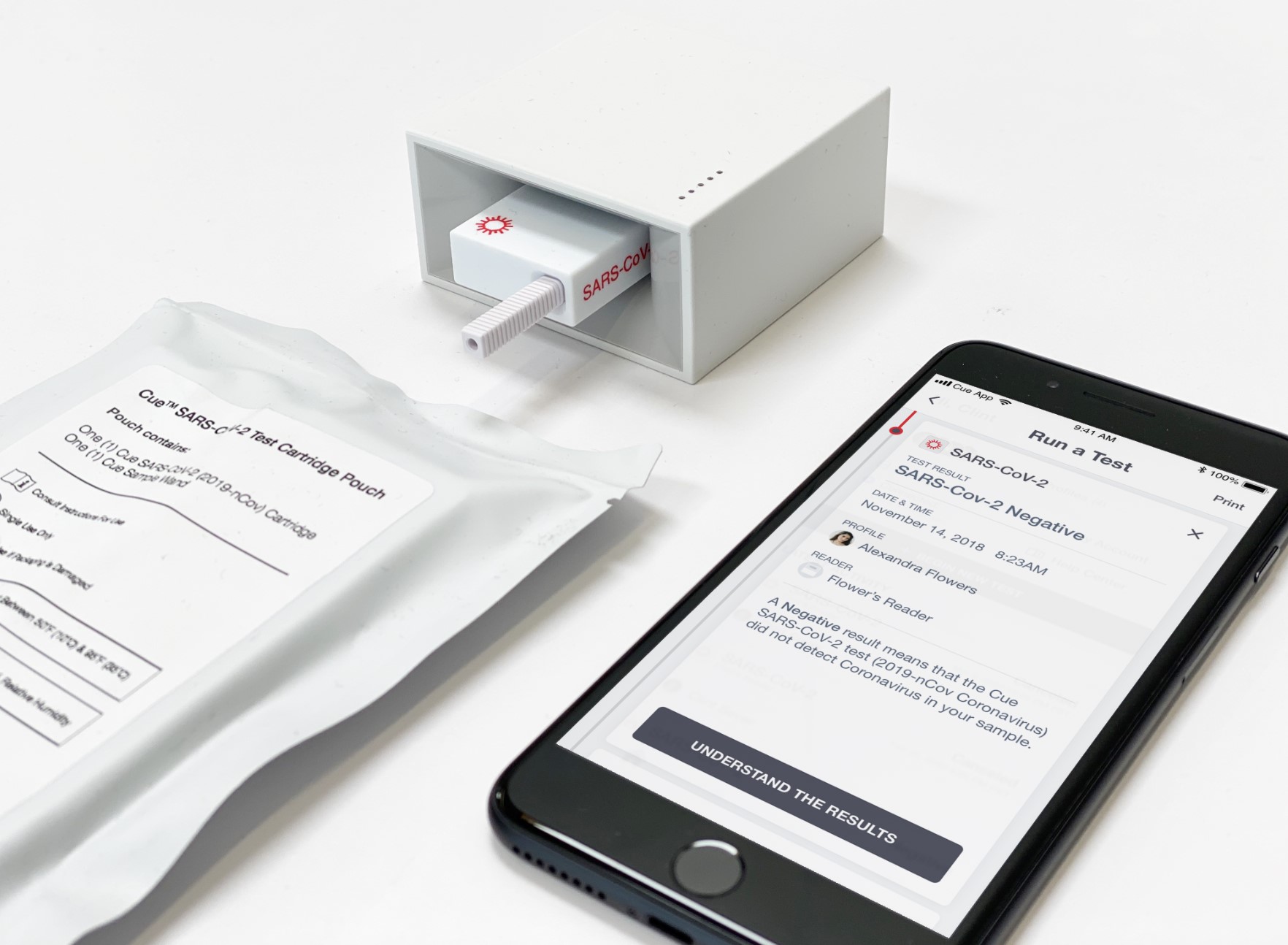
Cue Health received an emergency use authorization for its rapid molecular coronavirus test. The company is developing a portable testing platform that sends results to users’ smartphones. Photo credit: Cue Health
A San Diego-based startup won a $481 million contract from the Department of Health and Human Services (HHS) and the Department of Defense (DoD) to expand production of its rapid Covid-19 tests. Cue Health received an emergency use authorization in June for its point-of-care molecular test, which detects the RNA of SARS-CoV-2, the virus that causes Covid-19. The tests take about 20 minutes to process.
Cue said its tests are used in healthcare and business settings. Most notably, they were used by the NBA as it wrapped up its 2019-2020 season, with players or staff who entered the “NBA bubble” required to have two negative coronavirus tests.

The Funding Model for Cancer Innovation is Broken — We Can Fix It
Closing cancer health equity gaps require medical breakthroughs made possible by new funding approaches.
With the new funds, Cue will ramp up production to 100,000 Covid-19 test kits per day by March. Most of those tests will go to the government; HHS said it expects 6 million tests and 30,000 instruments as part of the contract.
“The Cue testing system is highly sensitive and specific, and nearly equivalent to the best large referral laboratory systems. It is FDA-authorized for use in anyone suspected of having Covid-19 by their health care provider,” Admiral Brett Giroir, an assistant secretary at HHS who is heading national testing efforts, said in a news release. “This investment will allow Cue Health, Inc. to expand its footprint and significantly scale up production, and by doing so enable this technology to be deployed throughout our testing ecosystem to benefit all Americans.”
Other competitors have also been developing rapid onsite tests. For example, Abbott’s 15-minute coronavirus test was touted by the White House, but came under fire after a Cleveland Clinic study found it only detected the virus in 85.2% of samples. Since then, Abbott has released interim data from a study showing its ID NOW rapid test picked up the virus 95% of the time.
For its part, Cue claims its Covid-19 test has a 98.7% sensitivity rate, meaning it identifies the virus most of the time, and a 97.6% specificity rate, indicating relatively few false positives. The HHS funding came after Mayo Clinic conducted a prospective study to evaluate the accuracy of its test, though it has not yet published the full results.

Health Executives on Digital Transformation in Healthcare
Hear executives from Quantum Health, Surescripts, EY, Clinical Architecture and Personify Health share their views on digital transformation in healthcare.
Cue’s system consists of a portable device, and disposable test cartridges specific to each disease. It can also send the results to users’ smartphones. Before the start of the pandemic, it was designing a system for at-home flu tests in conjunction with HHS’ Biomedical Advanced Research and Development Authority (BARDA). Earlier this year, the startup raised $100 million that it said would go toward trials for its FDA 510(k) submission.
While the system is designed with at-home use in mind, Cue’s Covid-19 tests must be administered by a medical professional, per the FDA’s emergency use authorization.
“Our vision in designing the Cue Health Monitoring System was to enable individuals to have more control over their health and lives by providing access to actionable, accurate health data in real-time,” Cue Health CEO Ayub Khattak wrote in an email. “To this end, the company plans to seek further authorization for home use.”








