
Rural Americans have difficulty accessing a promising cancer treatment
After settling in rural New Mexico, a cancer patient had to move 750 miles east and live in a trailer to access CAR-T treatment at the MD Anderson Cancer Center.

After settling in rural New Mexico, a cancer patient had to move 750 miles east and live in a trailer to access CAR-T treatment at the MD Anderson Cancer Center.
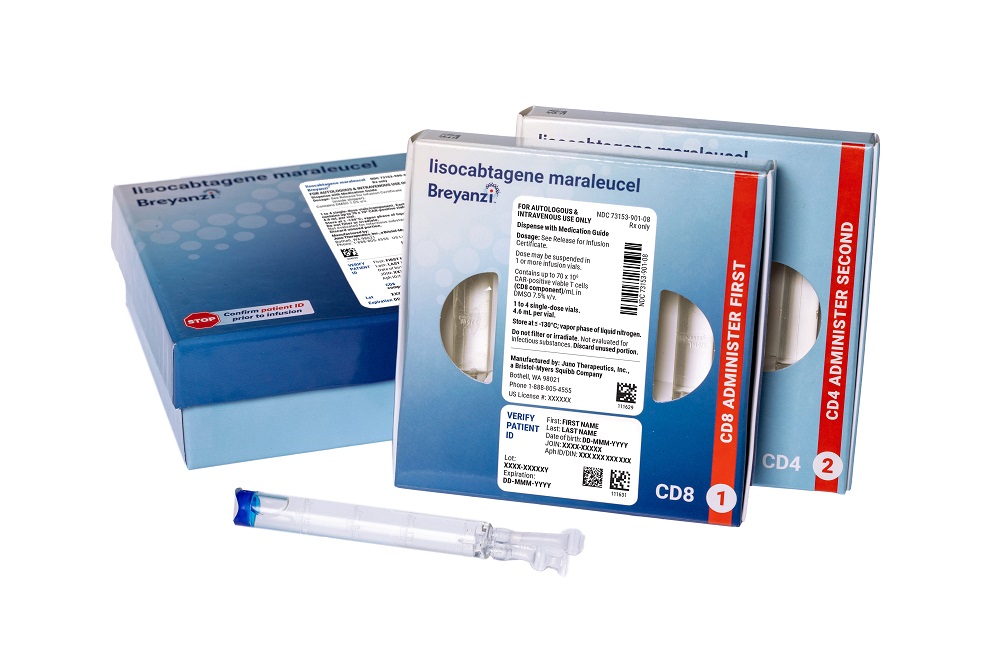
The new FDA approval for Breyanzi moves the Bristol Myers Squibb cancer cell therapy into an earlier line of treatment for an aggressive type of blood cancer. The decision also gives the pharmaceutical giant access to an additional pool of patients untapped by a rival cell therapy from Gilead Sciences.

As an oncologist, Kristen Hege first encountered cancer cell therapy research in the mid-1990s. Now as a Bristol Myers Squibb executive, she oversees efforts to improve the pharma giant’s first generation of cell therapies while also building a pipeline of next-generation treatments with better features and properties.
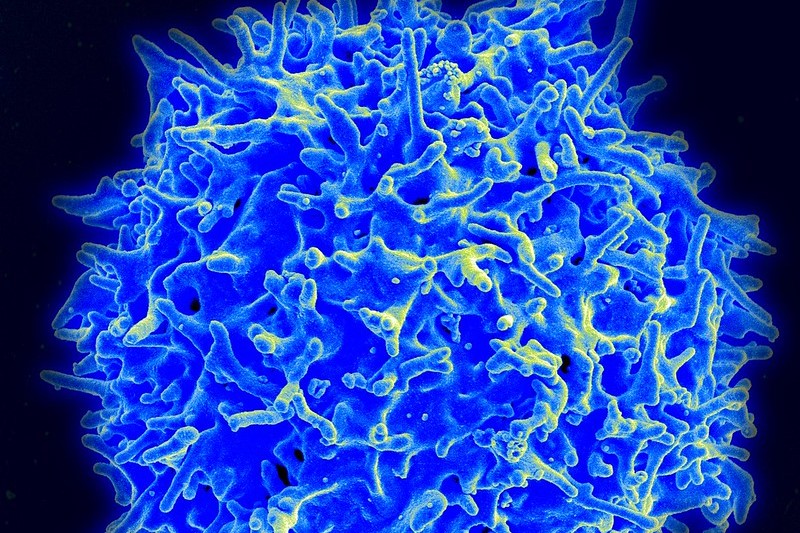
CAR T cell therapies for cancer still pose challenges in manufacturing, safety, and the ability to address solid tumors. A panel at the World Medical Innovation Forum discussed efforts to improve CAR T as well as new approaches for the next generation of cell therapies.

Approval of the Johnson & Johnson and Legend Biotech cell therapy, Carvykti, marks the second CAR T-cell therapy to clear the regulatory bar for multiple myeloma. The FDA approved Bristol Myers Squibb's cell therapy Abecma last year.
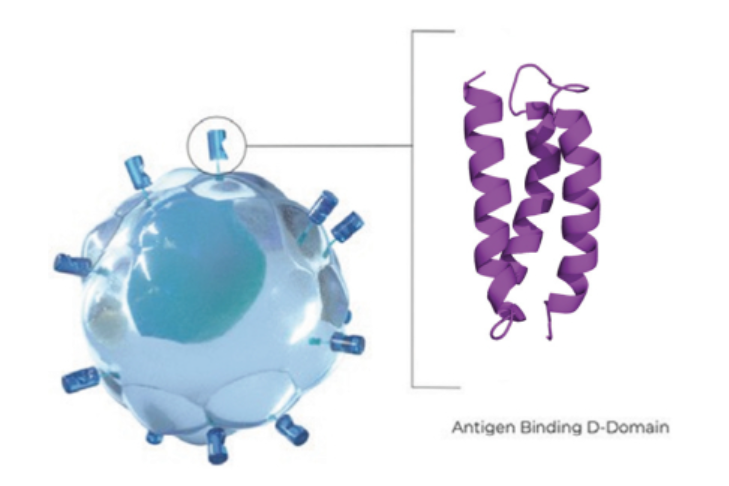
Clinical-stage Arcellx raised $123.8 million from its IPO, which the company will use to advance to a pivotal test for its lead program, a CAR T-cell therapy for multiple myeloma. Though Arcellx trails its large pharmaceutical rivals, the biotech contends its technology produces cell therapies with key advantages.
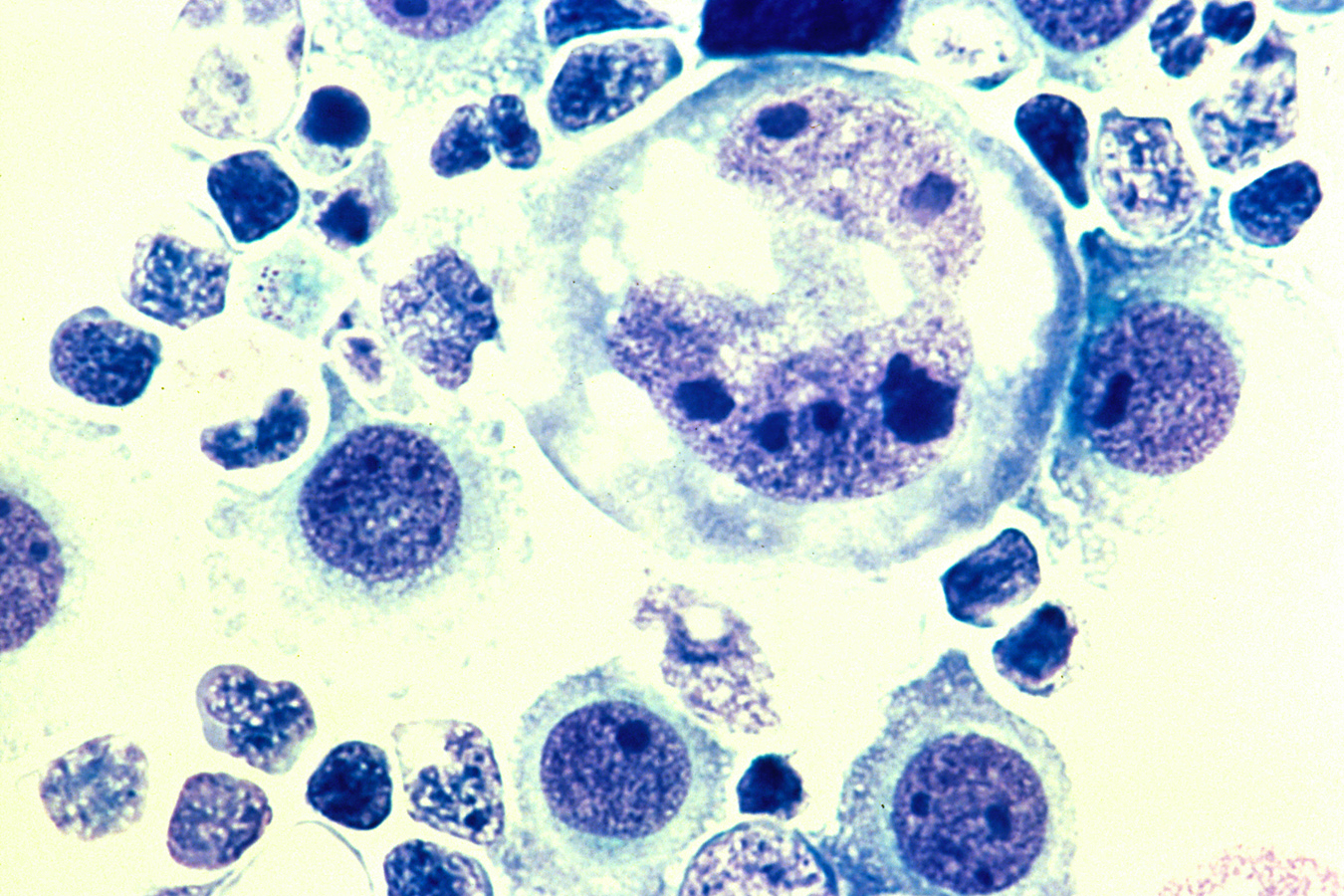
Despite the targeted approach of some cancer treatments, tumors can find ways to escape, leading patients to relapse. ImmPACT Bio will apply its Series B financing toward the development of cell therapies designed to prevent tumor escape.
The findings may make it possible to identify a subset of patients who experience poorer outcomes or more serious side effects, especially neurotoxicity.

The companies said Tuesday that the FDA had accepted their application for idecabtagene vicleucel, which they resubmitted in July after the agency initially refused to file it in May. The action date is in March of next year.
The company said Friday that it had submitted for approval of Yescarta in follicular and marginal zone lymphoma, based on data from the ZUMA-5 study that were presented at the 2020 ASCO meeting in May.

The company hopes to use the money to bring its T-cell antigen coupler cell therapy technology - currently in preclinical development - into human clinical trials. German drugmaker Bayer's venture capital arm led the round.
The company said the study of Kymriah in follicular lymphoma met its primary endpoint, though it did not disclose the trial data. It plans to submit approval applications to the FDA and EMA next year.

The companies' resubmission of their application Wednesday seeking approval for idecabtagene vicleucel was in line with the timeline they provided in May, when the FDA sent a refuse-to-file letter in response to their initial submission.

The agency approved Tecartus, previously developed under the name KTE-X19, as the first CAR-T therapy for mantle cell lymphoma. The company had previously won approval for another CAR-T, Yescarta. Tecartus is a similar product, but with a different manufacturing process.

The patient, who had been enrolled in the Phase I study at the higher dose level of UCARTCS1A, suffered a cardiac arrest, the causes of which are under investigation, the company said.