
RightEye’s eye-tracking system gets FDA clearance, based on competitor’s technology
Bethesda, Maryland-based RightEye has received a 510(k) clearance for its EyeQ eye-tracking platform using competitor SyncThink's technology as a predicate device.

Bethesda, Maryland-based RightEye has received a 510(k) clearance for its EyeQ eye-tracking platform using competitor SyncThink's technology as a predicate device.

Nature Medicine mouse study shows once-weekly GLP-1 receptor agonist slowed Parkinson's disease progression.

San Francisco-based firm, focusing on Parkinson's disease and amyotrophic lateral sclerosis, has raised $36 million to date.

The company will use the funding to develop mGlu4 PAMs licensed from Vanderbilt University.

A study by the Michael J Fox Foundation using Verily's Study Watch underscores the increasing use of wearables not only for Parkinson's disease research but also to identify ways to more accurately diagnose the neurodegenerative condition and to assess the impact of medication and other types of treatment interventions.
The funding is aimed at commercializing the device in the U.S. and other markets, according to the company's website citing The Australian Financial Review.

Boston Scientific's neurostimulation device Vercise is built on cochlear implant technology, allowing it to modulate impulses between multiple contacts.

Do doctors need a shot of empathy? Researchers at a Canadian digital healthcare marketing company think so, and say they can administer it wirelessly.

Some 3 1/2 years after having its hand slapped, 23andMe has received a green light from the FDA to market 10 direct-to-consumer genetic health risk reports for conditions such as Parkinson’s, late-onset Alzheimer’s, and celiac disease.

Championed as the first new chemical entity approved for Parkinson's disease in over a decade, safinamide is really part of an existing family of drugs that have been on the market for decades.

Companies developing deep brain stimulation and other neurostimulator technologies that can control and communicate signals to and from artificial body parts (i.e. limbs, eyeballs) are the latest players in the medical device sector.

The average cost of recruiting a trial subject via the targeted Facebook ads was $35, 96 percent less than the $800-per-person average through other means for the Michael J. Fox Foundation study.

Also, Bob Abraham, Pfizer's head of oncology research and development, talks about the ups and downs of drug development and Philadelphia becomes the first major city to pass a soda tax.
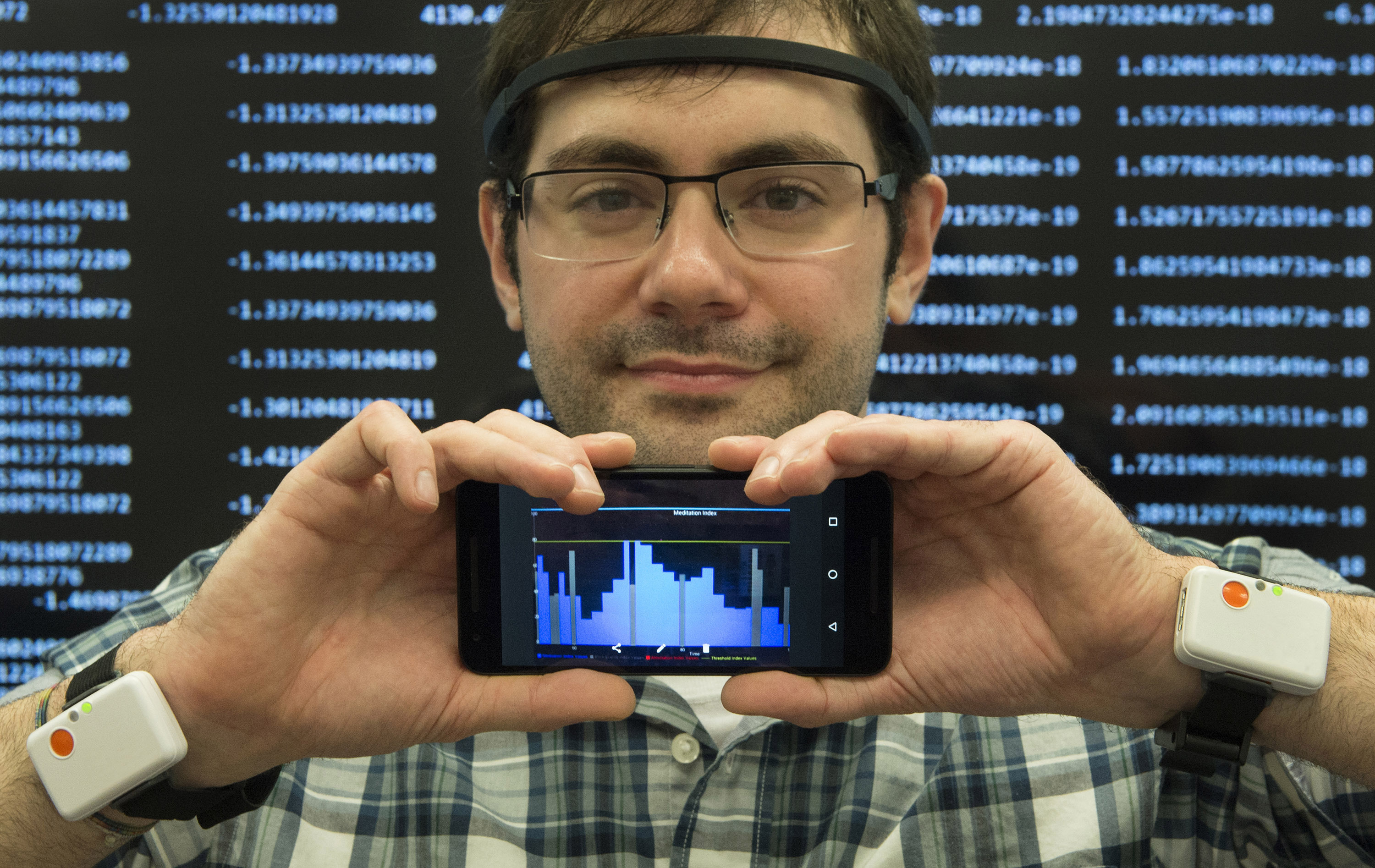
IBM and Pfizer will build a IoT ecosystem to measure the health and quality of life of Parkinson's patients.

Also, Aveo Pharmaceuticals will pay $4 million to settle charges with the SEC, and EMED Technologies said today that it is seeking a federal court injunction against competitor Repro-Med Systems.