The Food & Drug Administration launched a new initiative aimed at improving the safety of complex medical devices used by patients and caregivers in the home.
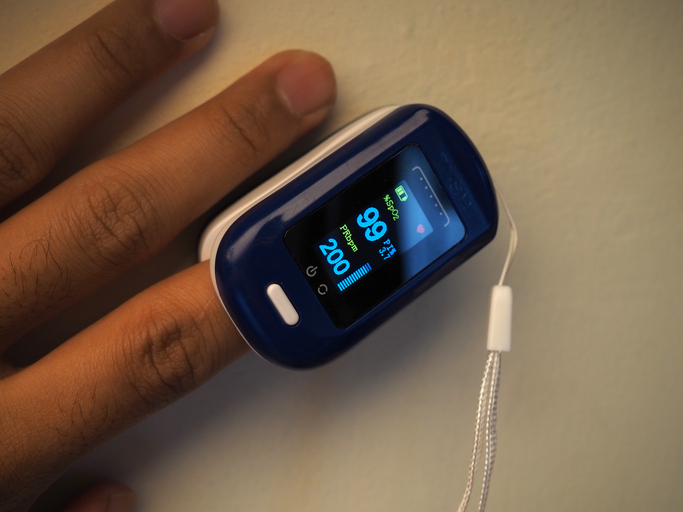
The federal watchdogs want to reduce the number of adverse events involving hemodialysis equipment, wound care, intravenous therapy devices and ventilators that patients and their caregivers are increasingly using at home, often without the supervision of doctors or other medical staff.
The FDA is planning a 10-month pilot program, beginning this summer, for manufacturers to voluntarily submit labeling of home-use medical devices to a central website. The agency believes posting the labeling data will improve access to information about the safe use of the devices for patients and caregivers. The FDA will also implement measures to enhance post-market surveillance of home-use devices, using “HomeNet,” part of its Medical Device Surveillance Network adverse event reporting program.

Unlocking Transparency in PBM Pricing
The TSX Venture Exchange has a strong history of helping early-stage health and life sciences companies raise patient capital for research and development.
The FDA also plans to develop guidelines for manufacturers marketing devices intended for home use, conduct post-market surveillance of those devices and develop educational resources for patients and caregivers. Because there’s no regulatory protocol for such devices, the guidelines for manufacturers will include:
- Recommendations for manufacturers to help them win pre-market approval or clearance, “including device testing with at-home caregivers and patients in a non-clinical setting,” according to a press release;
- Define the circumstances under which the agency might require that certain devices be labeled “that the device has not been cleared for use in the home;”
- And recommend post-market surveillance to monitor and address adverse events in the home.

When Investment Rhymes with Canada
Canada has a proud history of achievement in the areas of science and technology, and the field of biomanufacturing and life sciences is no exception.
The Massachusetts Medical Devices Journal is the online journal of the medical devices industry in the Commonwealth and New England, providing day-to-day coverage of the devices that save lives, the people behind them, and the burgeoning trends and developments within the industry.
This post appears through the MedCity Influencers program. Anyone can publish their perspective on business and innovation in healthcare on MedCity News through MedCity Influencers. Click here to find out how.