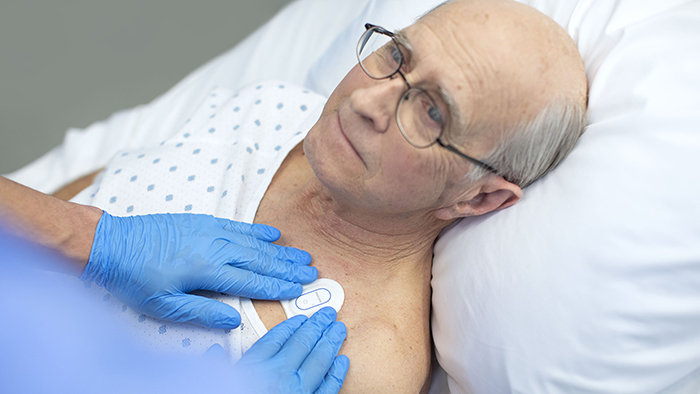
Philips received FDA 510(k) clearance for a wearable device that can monitor patients’ heart rate and respiratory rate. Photo credit: Philips
Long before the Covid-19 pandemic, Philips General Care head Ravi Kuppuraj and his team were working to develop a device that could quickly determine if a patient is beginning to deteriorate. They developed a disposable patch that could monitor a patient’s respiratory rate and heart rate after they are discharged from the ICU.
The two measurements are the top predictors of patient deterioration, and can be detected well before oxygen saturation levels drop or respiratory rates change.

Behavioral Health, Interoperability and eConsent: Meeting the Demands of CMS Final Rule Compliance
In a webinar on April 16 at 1pm ET, Aneesh Chopra will moderate a discussion with executives from DocuSign, Velatura, and behavioral health providers on eConsent, health information exchange and compliance with the CMS Final Rule on interoperability.
“Respiratory rate, it’s clearly a very good indicator for Covid-19,” Kuppuraj said in a phone interview. “It’s the right time for Covid. But it’s also a great solution for ambulatory care, remote care and the general detection of the deterioration of a patient’s health.”
While plenty of remote monitoring tools had been developed before the pandemic, hospitals are rapidly adopting them to reduce exposure to sick patients and conserve much-needed protective equipment. They can also be helpful in managing one of the puzzling aspects of Covid-19 — that some patients deteriorate frighteningly fast.
The device, called the Philips Biosensor BX100, received FDA 510(k) clearance in mid-May. It is currently approved to be used in a hospital setting, such as after a patient is discharged from the ICU, after surgery, or even after a patient is admitted to the hospital.
“It really allows the caregiver to start beginning to monitor a patient as soon as possible,” he said. “It’s really for use anywhere in the hospital that’s outside of critical care. … Once you come out of the critical care or intensive care settings of the hospital, what you’re really trying to do is monitor the patient for recovery or deterioration.”

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
Kuparraj said Philips began the FDA approval process in 2019.
“We got this in the nick of time,” he said.
The patch is meant to be worn on the sternum for up to five days. A single-use device, it doesn’t require any cables or charging. The biosensor has a built-in battery and connects to a hospital’s back-end infrastructure via Bluetooth.
Specifically, it’s designed to connect with Philips’ Intellivue Guardian early warning scoring system, which can connect into a hospital’s electronic health record system and alert physicians on a mobile device if they might need to escalate a patient’s care.
Kuparraj said Philips had been working to develop the entire system for about three years, including the sensors and the back-end software. Part of that process included determining what would make sense with physician workflows and provide meaningful information on a patient’s health.
“Like anything in healthcare, it’s been a journey that has taken a significant amount of effort, thought and time,” he said.












