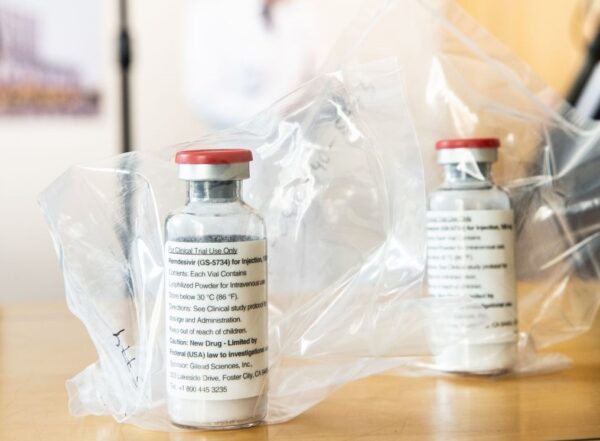
A large U.S. drugmaker is partnering with Gilead Sciences to manufacture the latter company’s antiviral drug for treating Covid-19.
New York-based Pfizer said Friday that it had made a multi-year agreement with Foster City, California-based Gilead to manufacture remdesivir, the antiviral drug that received an emergency use authorization from the Food and Drug Administration for patients hospitalized with Covid-19 in May. Pfizer will produce the drug at a factory in McPherson, Kansas. The drug is not yet approved, but Gilead has been referring to it under the brand name Veklury.

With the Rise of AI, What IP Disputes in Healthcare Are Likely to Emerge?
Munck Wilson Mandala Partner Greg Howison shared his perspective on some of the legal ramifications around AI, IP, connected devices and the data they generate, in response to emailed questions.
“From the beginning, it was clear that no one company or innovation would be able to bring an end to the Covid-19 crisis,” Pfizer CEO Albert Bourla said in a statement. “Pfizer’s agreement with Gilead is an excellent example of members of the innovation ecosystem working together to deliver medical solutions.”
In its second-quarter 2020 earnings on July 30, Gilead said that it was working with partners in North America, Asia and Europe in order to supplement its internal manufacturing of remdesivir while also streamlining its own processes. The company expects to manufacture more than 2 million treatment courses by the end of this year and several million more in 2021.
The news comes as concerns have emerged of whether there will be sufficient supplies of remdesivir for the world.
In July, the Department of Health and Human Services said it had acquired all the supplies of the drug for that month and 90% of supplies for August and September, leaving other developed nations effectively without access. While many developing countries have access to generic versions of the drug, World Trade Organization rules complicate wealthier nations’ ability to access them. However, Gilead said in its second-quarter earnings that it had agreed last month with the European Commission to allow the EC to centrally purchase the drug over the next few months under emergency provisions in order to allocate it to European Union member countries and the U.K.
The drug received the EUA in May on the basis of a Phase III randomized, placebo-controlled trial showing it produced a statistically significant improvement in the time for patients hospitalized with Covid-19 to recover. Data for patient survival were not statistically significant despite showing a trend in favor of the drug. Because remdesivir is administered intravenously over 30 minutes to two hours, its use is limited to the inpatient setting. However, Gilead has an inhaled version of the drug it is testing in a Phase I study among healthy volunteers.
Several other companies have been developing antiviral treatments as well, such as Regeneron Pharmaceuticals, whose REGN-COV2 is a two-antibody cocktail developed as a prophylactic and as a treatment.
Photo: Ulrich Perrey, Pool/AFP, via Getty Images














