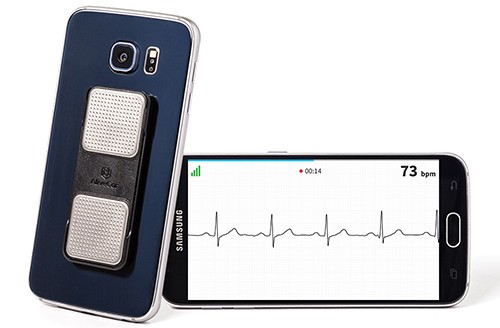
As more companies turn an eye to at-home ECG measurements, AliveCor received clearance from the Food and Drug Administration for its newest suite of algorithms. The Mountain View, Calif.-based startup has developed a portable electrocardiogram (ECG) that pairs with a mobile app.
As Fitbit and Apple land clearances for algorithms to detect atrial fibrillation, AliveCor is pursuing additional clinical indications.
Last year, it got FDA clearance for its six-lead ECG device, which allows cardiologists to view electrical activity in the heart from six perspectives, giving them the ability to detect a broader range of heart conditions. Users hold a small device between their fingers for 30 seconds to receive a reading.
Its most recent clearance will reduce the number of “unclassified” readings and improve the sensitivity and specificity of its “normal” and “atrial fibrillation” algorithms. It also will make the device better at detecting premature beats, which can be another reason patients feel palpitations. The new algorithms include:
- Sinus rhythm with premature ventricular contractions, a common occurrence where extra heartbeats originate in the bottom chamber of the heart, sometimes leading to the sensation of the heart “skipping a beat.”
- Sinus rhythm with supraventricular ectopy, a premature heartbeat originating in the top chamber of the heart.
- Sinus rhythm with wide QRS, which could indicate that the activation of the bottom chamber of the heart is taking longer than expected.
“This suite of algorithms and visualizations will provide the platform for delivery of new consumer and professional service offerings beyond AFib, by allowing a much wider range of cardiac conditions to be determined on a personal ECG device,” AliveCor CEO Priya Abani said in a news release.

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
The news comes just a week after AliveCor raised $65 million in series E funding. It plans to use the funds to grow its remote cardiology program in the U.S. and overseas.
In addition to its devices, the company recently rolled out virtual care services through its app, including the ability to have a board-certified cardiologist review ECG results every three months.
Photo credit: AliveCor












