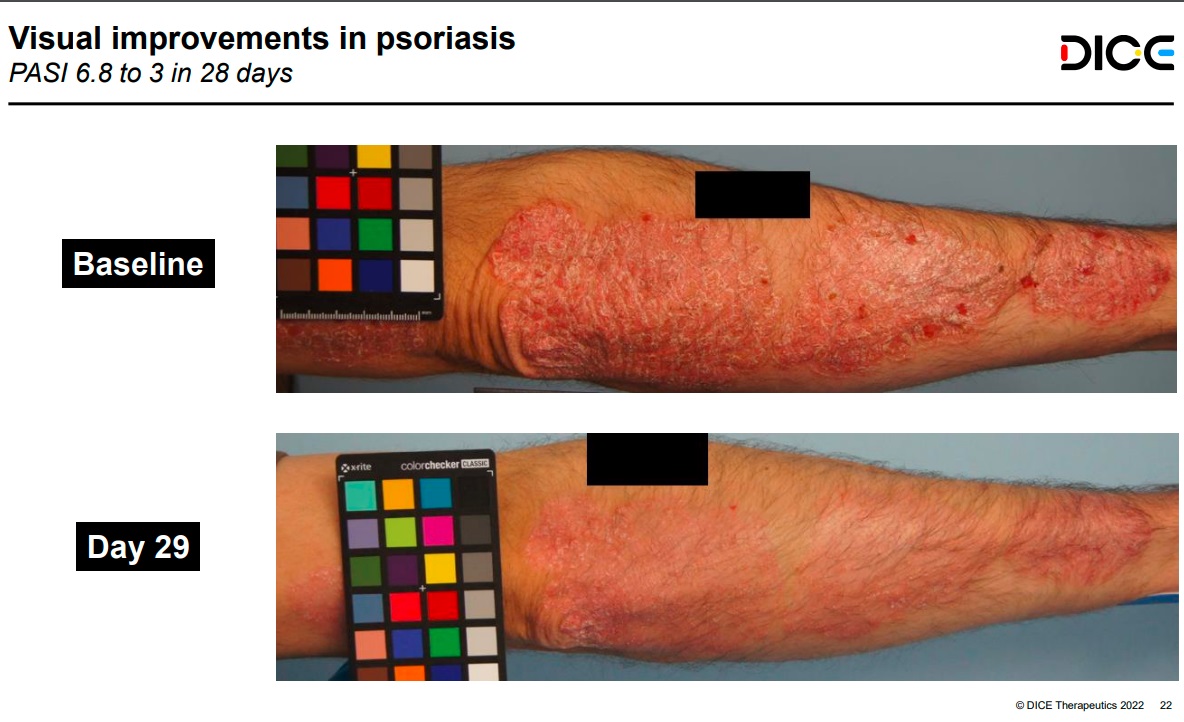
An experimental Dice Therapeutics drug in development for treating psoriasis now has its first human data showing it reduces the signs and symptoms of the inflammatory skin disorder. The preliminary results announced Tuesday are from a small Phase 1 study, but they indicate that Dice’s small molecule can hit the same target as currently available biologic drugs, helping to build a case for an oral alternative to blockbuster medicines that must be injected or infused.

With the Rise of AI, What IP Disputes in Healthcare Are Likely to Emerge?
Munck Wilson Mandala Partner Greg Howison shared his perspective on some of the legal ramifications around AI, IP, connected devices and the data they generate, in response to emailed questions.
The Dice drug, DC-806, is designed to block IL-17, a pro-inflammatory signaling protein. Following dose-ascending tests in healthy volunteers, two doses were selected for testing in psoriasis patients. Across both the high dose (8 patients) and low dose (13 patients) tested, South San Francisco-based Dice reported an average 43.7% reduction in scores according to a scale used to assess the severity of psoriasis symptoms. That compares to a 13.3% average reduction in the 11 patients in the placebo group.
Investors welcomed the early results for Dice. Shares of the biotech opened Tuesday at $44.18 apiece, up more than 79% from Friday’s closing price.
IL-17 can be blocked by antibody drugs, such as Novartis’s Cosentyx and Eli Lilly’s Taltz. Both blockbuster medicines are approved for treating psoriasis, among other inflammatory disorders. While oral drugs are available for treating psoriasis, there are no FDA-approved oral medications that target IL-17. For chronic conditions such as psoriasis, patients prefer the convenience of pills to injections or infusions.
Dice said in an investor presentation that its twice-daily pill is designed to block the same biochemical step as the IL-17-targeting antibody drugs. But like other autoimmune disorder drugs that tamp down a part of the immune system, use of IL-17 antibodies raises the risk that a patient develops serious infections. Dice reported that all adverse events related to the treatment were classified as mild or moderate. Furthermore, there was no dose-dependent trend in the frequency, severity, or type of such events.
Dice’s clinical data for its lead program come a year after the company raised more than $200 million from its IPO. The biotech designs its small molecules with a proprietary technology platform called Delscape. With the encouraging results from Phase 1 testing, Dice now plans to see how well the drug works in a larger group of patients over a longer period of time. The company is planning to advance DC-806 to a Phase 2b clinical trial in the first half of 2023. That 12-week study will enroll adults with moderate-to-severe psoriasis who will receive multiple doses that will be compared against a placebo. The goal is to evaluate safety and efficacy at 12 weeks, which will in turn help the company determine the dose to advance to Phase 3 testing.
DC-806’s results showing that it can hit IL-17 in a safe manner has Dice thinking of using the same drug to address other disorders driven by that protein. Autoimmune and inflammatory conditions associated with IL-17 include rheumatoid arthritis, multiple sclerosis, Crohn’s disease, and lupus.
“We believe these data not only support further development of DC-806 as a potential best-in-class oral therapy for psoriasis, but also may unlock additional IL-17-mediated disease indications given DC-806’s excellent safety profile, strong [pharmacokinetic] data, and robust, dose-dependent target engagement,” CEO Kevin Judice said in a prepared statement. “More broadly, we believe these data validate our Delscape platform as we work to advance more oral small molecule therapeutics to address other validated protein-protein interaction targets in immunology and other therapeutic areas.”
Graphic from Dice Therapeutics investor presentation














