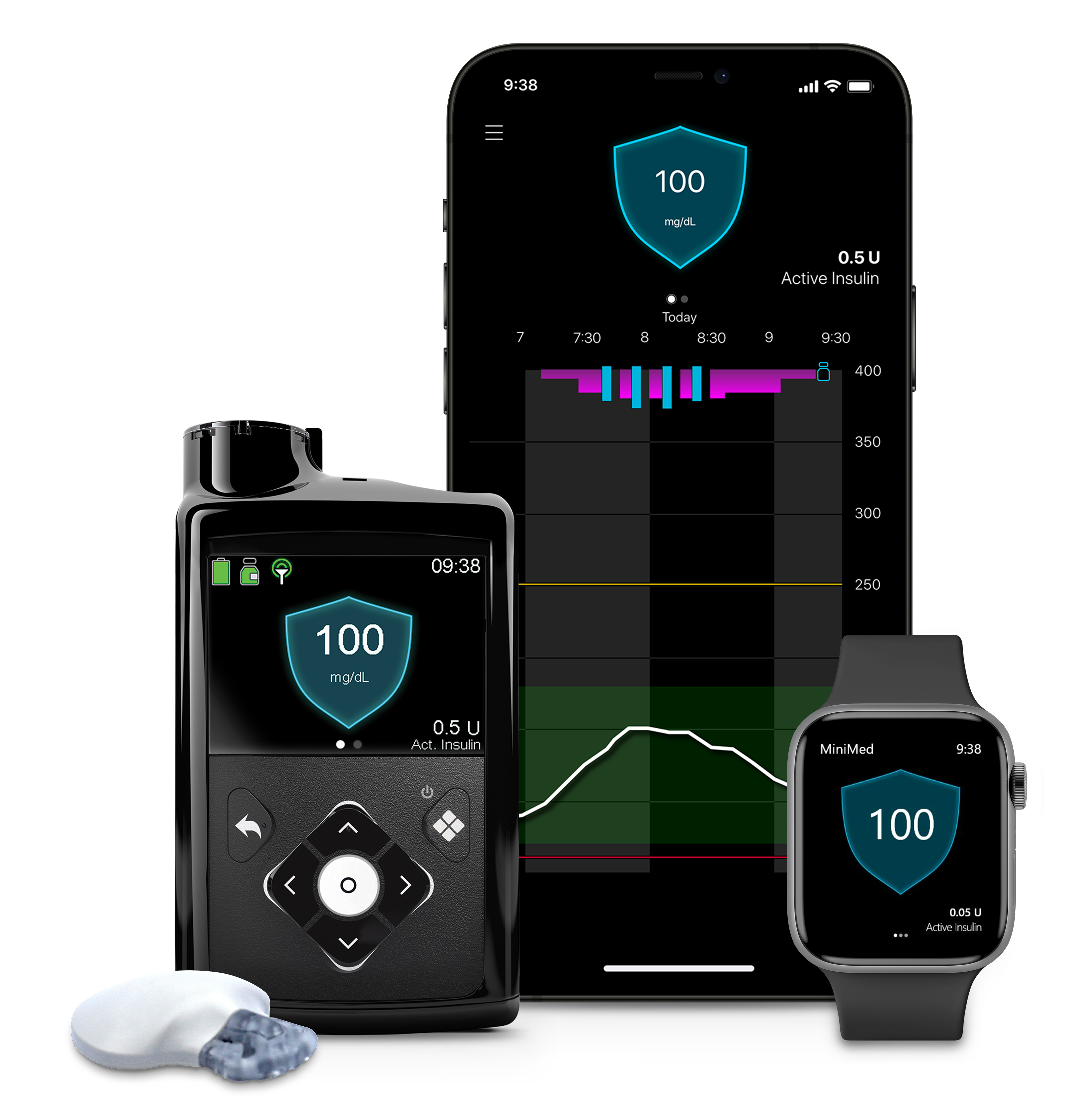
Late last week, medical device giant Medtronic received FDA approval for its MiniMed 780G system. The device is an insulin pump that automatically adjusts and corrects type 1 diabetes patients’ glucose levels every five minutes.
The news comes two years after Medtronic submitted the system for FDA approval and three years after the company began selling the product in Europe.
“We believe that diabetes management today is too complicated and intrusive. Growth in continuous glucose monitoring has been a good development, but we don’t believe that it is enough. It’s good to have data from the sensor, but you really drive outcomes with action,” said Que Dallara, president of Medtronic’s diabetes division, in a recent interview.
The MiniMed 780G pump seeks to simplify patients’ management of their type 1 diabetes by working together with Medtronic’s Guardian 4 continuous glucose monitoring sensor and SmartGuard technology for insulin adjustments.
The system eliminates the need for finger pricks and calibrations, as well as automatically delivers basal insulin adjustments and autocorrections to a set target, Dallara declared.
“We want to do as much as we can to push diabetes to the background and make our customers happy,” she pointed out.
The MiniMed 780G pump takes glucose readings from the Guardian 4 sensor every five minutes and then makes automatic adjustments and corrections to insulin delivery, Dallara explained. If a patient’s sugar levels are trending high, the system automatically provides more insulin. If sugar levels are trending low, the system automatically reduces the amount of insulin delivered.
With its new FDA approval, Medtronic can now sell the MiniMed 780G system in the U.S. for use in patients with type 1 diabetes who are seven years of age or older. And patients who use the company’s MiniMed 770G system are now eligible to upgrade their device to the MiniMed 780G through a no-cost, remote software upgrade.
But MiniMed devices are not the only insulin pumps on the market — there’s also devices made by companies like Insulet and Tandem Diabetes Care.
“What sets the MiniMed 780G system apart from other automated insulin delivery systems on the market is the real-time auto-corrections and overall simplification of the user experience,” Dallara declared.
Medtronic’s system is the only one on the market that can provide automatic adjustments and corrections every five minutes to support users when they occasionally forget to dose themselves with insulin or underestimate the number of carbs in their meal, she argued.
Additionally, the MiniMed 780G pump features a lower glucose target setting than the other automated insulin pumps on the market — as low as 100 mg/dL — Dallara said. This glucose target setting more closely mirrors the average glucose levels of someone who does not live with diabetes, she pointed out.
“With this setting, the pump will ‘treat to target’ and will automatically deliver basal insulin adjustments and autocorrections to a set target,” she explained.
The MiniMed 780G system is also the only pump with an infusion set that can be worn for up to seven days, which doubles wear time, Dallara added.
Photo: Medtronic