An Eli Lilly Alzheimer’s disease drug that the FDA had turned down due to insufficient data from a mid-stage study now has clinical trial results showing the amyloid plaque-busting therapy met the goals of a larger and longer Phase 3 test, reviving hopes that the therapy can offer yet another new treatment option for the neurodegenerative disorder.
According to the preliminary data reported Wednesday, compared to a placebo, Lilly drug donanemab led to a 35% slowing of decline, meeting the main goal of the 18-month study. The experimental therapy also met secondary goals assessing various measures of cognitive and functional decline. Based on these results, the Indianapolis-based pharmaceutical giant said it would proceed “as quickly as possible” with regulatory submissions around the world, including an FDA filing by the end of June.

With the Rise of AI, What IP Disputes in Healthcare Are Likely to Emerge?
Munck Wilson Mandala Partner Greg Howison shared his perspective on some of the legal ramifications around AI, IP, connected devices and the data they generate, in response to emailed questions.
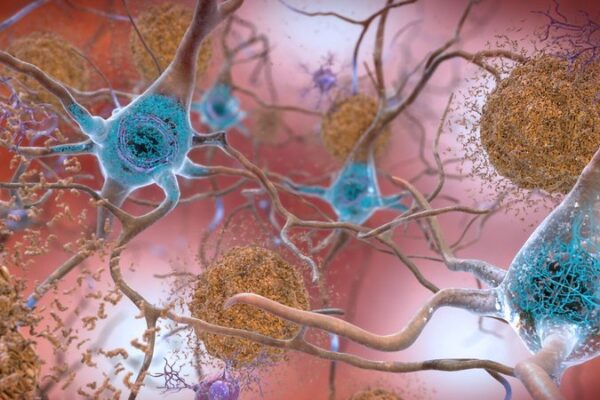
Donanemab is an antibody designed to target and break up plaques of amyloid that form in the brains of Alzheimer’s patients. It belongs to the same class of therapies as Aduhelm, the Biogen drug approved by the FDA in 2021, and Eisai’s Leqembi, which the agency approved earlier this year.
The intravenously infused Lilly drug was evaluated in a Phase 3 study that enrolled 1,736 patients who were selected based on cognitive assessments as well as imaging to detect the presence of both amyloid and tau, proteins whose buildup in the brain is a hallmarks of the neurodegenerative disease’s progression. Though the Phase 3 study followed patients for 18 months, participants completed their course of treatment once imaging scans showed they reached a prespecified level of amyloid plaque clearance.
A similar study design led to positive Phase 2 results that were the basis for an application seeking accelerated FDA approval. But in January, the FDA rejected the submission, telling Lilly it needed data from at least 100 patients followed for at least 12 consecutive months. Because patients stopped dosing of donanemab once plaque clearance was achieved, Lilly said many study participants were able to stop receiving the drug as early as six months, leaving the company short of the patient number threshold set by the FDA.
The Phase 3 study stratified participants according to their tau levels. For the analysis of donanemab’s efficacy, the study focused on patients whose tau levels and clinical Alzheimer’s symptoms were classified as intermediate. A total of 1,182 patients fit those criteria. This is the group that achieved the main goal as measured by an assessment called integrated Alzheimer’s disease Rating Scale (iADRS). This scale measures a patient’s cognition as well as the ability to perform daily tasks, such as managing finances, driving, pursuing hobbies, and talking about current events.
Patients in this primary analysis group also met a secondary goal, showing a 36% slowing in decline over 18 months according to Clinical Dementia Rating-Sum of Boxes (CDR-SB). That’s the same scale used to assess both Aduhelm and Leqembi in their respective Phase 3 studies. According to this scale, Lilly said 47% of patients who received its drug showed no decline at one year compared to 29% of those who were given a placebo.
Cross-trial comparisons can be tricky and even misleading, but the CDR-SB results for the Lilly drug top the 27% slowing in decline that Eisai reported for Leqembi in Phase 3 testing. The Eisai drug’s January regulatory decision was an accelerated approval based on Phase 2 data. The Japanese drugmaker immediately filed an application seeking full FDA approval based on the Phase 3 data. The FDA has set a July 6 target date for deciding whether to award Leqembi full approval.
While donanemab’s preliminary results help to build its case as a new therapeutic option, treatment with the Lilly drug also led to incidents of amyloid-related imaging abnormalities (ARIA), swelling and bleeding in the brain that is a known complication of this class of Alzheimer’s drugs. The brain swelling was observed in 24% of treated patients while the bleeding complication was reported in 31.4%. Lilly said the incidence of serious ARIA was 1.6%, including two patients whose death was attributed to the complication. A third study participant died after an incident of serious ARIA, Lilly said.
“We are encouraged by the potential clinical benefits that donanemab may provide, although like many effective treatments for debilitating and fatal diseases, there are associated risks that may be serious and life-threatening,” Mark Mintun, group vice president neuroscience research & development at Lilly, said in a prepared statement. “We note that these results suggest that people in the early pathological stage of disease could be the most responsive to therapeutics targeting amyloid.”
The preliminary results released Wednesday have not yet been reviewed by the scientific community. Lilly said full results will be presented in July at the Alzheimer’s Association International Conference and submitted for publication in a peer-reviewed scientific journal.
Public domain image by Flicker user NIH Image Gallery














